Recovery of metals with carbon monoxide. Methods for obtaining metals. What will we do with the received material?
In his daily life is surrounded by various metals. Most of the items we use contain these chemicals. This all happened because people found a variety of ways to obtain metals.
What are metals
Inorganic chemistry deals with these valuable substances for people. Obtaining metals allows a person to create more and more perfect technology that improves our lives. What are they? Before considering the general methods for obtaining metals, it is necessary to understand what they are. Metals are a group of chemical elements in the form of simple substances with characteristic properties:
Thermal and electrical conductivity;
High plasticity;
Glitter.
A person can easily distinguish them from other substances. A characteristic feature of all metals is the presence of a special brilliance. It is obtained by reflecting incident light rays onto a surface that does not transmit them. Shine is a common property of all metals, but it is most pronounced in silver.
To date, scientists have discovered 96 such chemical elements, although not all of them are recognized by official science. They are divided into groups depending on their characteristic properties. So the following metals are distinguished:
Alkaline - 6;
Alkaline earth - 6;
Transitional - 38;
Lungs - 11;
Semimetals - 7;
Lanthanides - 14;
Actinides - 14.
Obtaining metals
In order to make an alloy, it is necessary first of all to obtain metal from natural ore. Native elements are those substances that are found in nature in a free state. These include platinum, gold, tin, mercury. They are separated from impurities mechanically or with the help of chemical reagents.
Other metals are mined by processing their compounds. They are found in various fossils. Ores are minerals and rocks, which include metal compounds in the form of oxides, carbonates or sulfides. To obtain them, chemical processing is used.
Recovery of oxides with coal;
Obtaining tin from tin stone;
Burning sulfur compounds in special furnaces.
To facilitate the extraction of metals from ore rocks, various substances called fluxes are added to them. They help remove unwanted impurities such as clay, limestone, sand. As a result of this process, low-melting compounds called slags are obtained.
In the presence of a significant amount of impurities, the ore is enriched before smelting the metal by removing a large part of the unnecessary components. The most widely used methods for this treatment are flotation, magnetic and gravity methods.

alkali metals
Mass production of alkali metals is a more complex process. This is due to the fact that they are found in nature only in the form of chemical compounds. Since they are reducing agents, their production is accompanied by high energy costs. There are several ways to extract alkali metals:
Lithium can be obtained from its oxide in a vacuum or by electrolysis of its chloride melt, which is formed during the processing of spodumene.
Sodium is extracted by calcining soda with coal in tightly closed crucibles or by electrolysis of a chloride melt with the addition of calcium. The first method is the most laborious.
Potassium is obtained by electrolysis of a melt of its salts or by passing sodium vapor through its chloride. It is also formed by the interaction of molten potassium hydroxide and liquid sodium at a temperature of 440°C.
Cesium and rubidium are mined by reducing their chlorides with calcium at 700-800°C or zirconium at 650°C. Obtaining alkali metals in this way is extremely energy intensive and expensive.
Differences between metals and alloys
There is practically no fundamentally clear boundary between metals and their alloys, since even the purest, simplest substances have some proportion of impurities. So what is the difference between them? Almost all metals used in industry and in other sectors of the national economy are used in the form of alloys, obtained purposefully by adding other components to the main chemical element.
Alloys
The technique requires a variety of metallic materials. At the same time, pure chemical elements are practically not used, since they do not have the properties necessary for people. For our needs, we have invented different ways to obtain alloys. This term refers to a macroscopically homogeneous material that consists of 2 or more chemical elements. In this case, metal components predominate in the alloy. This substance has its own structure. In alloys, the following components are distinguished:
A base consisting of one or more metals;
Small additions of modifying and alloying elements;
Unremoved impurities (technological, natural, random).
It is metal alloys that are the main structural material. There are more than 5000 of them in technology.

Despite such a variety of alloys, those based on iron and aluminum are of the greatest importance for people. They are the most common in everyday life. The types of alloys are different. Moreover, they are divided according to several criteria. So various methods of manufacturing alloys are used. According to this criterion, they are divided into:
Cast, which are obtained by crystallization of the melt of mixed components.
Powder, created by pressing a mixture of powders and subsequent sintering at high temperature. Moreover, often the components of such alloys are not only simple chemical elements, but also their various compounds, such as titanium or tungsten carbides in hard alloys. Their addition in certain quantities changes the materials.
Methods for obtaining alloys in the form of a finished product or blank are divided into:
Foundry (silumin, cast iron);
Deformable (steels);
Powder (titanium, tungsten).

Alloy types
Methods for obtaining metals are different, while the materials made thanks to them have different properties. In the solid state of aggregation, alloys are:
Homogeneous (homogeneous), consisting of crystals of the same type. They are often referred to as single phase.
Heterogeneous (heterogeneous), called multiphase. When they are obtained, a solid solution (matrix phase) is taken as the base of the alloy. The composition of heterogeneous substances of this type depends on the composition of its chemical elements. Such alloys may contain the following components: solid solutions of interstitial and substitution, chemical compounds (carbides, intermetallides, nitrides), crystallites of simple substances.
Alloy properties
Regardless of which methods of obtaining metals and alloys are used, their properties are completely determined by the crystal structure of the phases and the microstructure of these materials. Each of them are different. The macroscopic properties of alloys depend on their microstructure. In any case, they differ from the characteristics of their phases, which depend solely on the crystal structure of the material. The macroscopic homogeneity of heterogeneous (multiphase) alloys is obtained as a result of a uniform distribution of phases in the metal matrix.
The most important property of alloys is weldability. Otherwise, they are identical to metals. So, alloys have thermal and electrical conductivity, ductility and reflectivity (shine).

Varieties of alloys
Various methods of obtaining alloys have allowed man to invent a large number of metallic materials with different properties and characteristics. According to their purpose, they are divided into the following groups:
Structural (steel, duralumin, cast iron). This group also includes alloys with special properties. So they are distinguished by intrinsic safety or anti-friction properties. These include brass and bronze.
For pouring bearings (babbitt).
For electric heating and measuring equipment (nichrome, manganin).
For the production of cutting tools (will win).
In production, people also use other types of metallic materials, such as low-melting, heat-resistant, corrosion-resistant and amorphous alloys. Magnets and thermoelectrics (telurides and selenides of bismuth, lead, antimony, and others) are also widely used.
Iron alloys
Almost all the iron smelted on Earth is directed to the production of simple iron. It is also used in the production of pig iron. Iron alloys have gained their popularity due to the fact that they have properties that are beneficial to humans. They were obtained by adding various components to a simple chemical element. So, despite the fact that various iron alloys are made on the basis of one substance, steels and cast irons have different properties. As a result, they find a variety of applications. Most steels are harder than cast iron. Various methods for obtaining these metals make it possible to obtain different grades (brands) of these iron alloys.

Improvement of alloy properties
By fusing certain metals and other chemical elements, materials with improved characteristics can be obtained. For example, pure aluminum is 35 MPa. Upon receipt of an alloy of this metal with copper (1.6%), zinc (5.6%), magnesium (2.5%), this figure exceeds 500 MPa.
By combining various chemical substances in different proportions, metal materials with improved magnetic, thermal or electrical properties can be obtained. The main role in this process is played by the structure of the alloy, which is the distribution of its crystals and the type of bonds between atoms.
Steels and cast irons
These alloys are obtained by and carbon (2%). In the production of alloyed materials, nickel, chromium, and vanadium are added to them. All ordinary steels are divided into types:
Low-carbon (0.25% carbon) is used for the manufacture of various structures;
High-carbon (more than 0.55%) is intended for the production of cutting tools.
Various grades of alloyed steels are used in mechanical engineering and other products.
An alloy of iron with carbon, the percentage of which is 2-4%, is called cast iron. This material also contains silicon. Various products with good mechanical properties are cast from cast iron.

Non-ferrous metals
In addition to iron, other chemical elements are used to make various metallic materials. As a result of their combination, non-ferrous alloys are obtained. In people's lives, materials based on:
Copper, called brass. They contain 5-45% zinc. If its content is 5-20%, then brass is called red, and if 20-36% - yellow. There are alloys of copper with silicon, tin, beryllium, aluminum. They are called bronzes. There are several types of such alloys.
Lead, which is a common solder (tretnik). In this alloy, 2 parts of tin fall on 1 part of this chemical. In the production of bearings, babbitt is used, which is an alloy of lead, tin, arsenic and antimony.
Aluminum, titanium, magnesium and beryllium, which are light non-ferrous alloys with high strength and excellent mechanical properties.
How to get
The main methods for obtaining metals and alloys:
Foundry, in which the solidification of various molten components occurs. To obtain alloys, pyrometallurgical and electrometallurgical methods of obtaining metals are used. In the first variant, thermal energy obtained in the process of fuel combustion is used to heat the raw material. The pyrometallurgical method produces steel in open-hearth furnaces and cast iron in blast furnaces. With the electrometallurgical method, the raw materials are heated in induction or electric arc furnaces. At the same time, the raw material is disintegrated very quickly.
Powder, in which the powders of its components are used to make the alloy. Thanks to pressing, they are given a certain shape, and then sintered in special furnaces.
The significant chemical activity of metals (interaction with atmospheric oxygen, other non-metals, water, salt solutions, acids) leads to the fact that they are found in the earth's crust mainly in the form of compounds: oxides, sulfides, sulfates, chlorides, carbonates, etc. In free form, there are metals located in the series of voltages to the right of hydrogen (Ag, Hg, Pt, Au, Cu), although much more often copper and mercury can be found in nature in the form of compounds.
Minerals and black rocks containing metals and their compounds, from which the extraction of pure metals is technically possible and economically feasible, are called ores.
Obtaining metals from ores is the task of metallurgy.
Metallurgy- this is both the science of industrial methods for obtaining metals from ores, and a branch of industry.
Any metallurgical process is a process of reduction of metal ions with the help of various reducing agents. Its essence can be expressed as follows:
M n+ + ne−→M
To implement this process, it is necessary to take into account the activity of the metal, select a reducing agent, consider technological feasibility, economic and environmental factors.
In accordance with this, there are the following methods for obtaining metals:
pyrometallurgical;
Hydrometallurgical;
Electrometallurgical.
Pyrometallurgy
Pyrometallurgy is the recovery of metals from ores at high temperatures using carbon, carbon monoxide (II), hydrogen, metals - aluminum, magnesium.
For example, tin is reduced from cassiterite SnO 2, and copper is reduced from cuprite Cu 2 O
calcination with coal (coke):
SnO 2 + 2C \u003d Sn + 2CO; Cu 2 O + C \u003d 2Cu + CO
Sulfide ores are preliminarily roasted with air access, and then the resulting oxide is reduced with coal:
2ZnS + 30 2 \u003d 2ZnO + 2SO 2; ZnO + C \u003d Zn + CO
sphalerite (zinc blende)
Metals are also isolated from carbonate ores by calcination with coal, since carbonates decompose when heated, turning into oxides, and the latter are reduced by coal:
FeCO3 \u003d FeO + CO 2; FeO + C = Fe + CO
siderite (spar ironstone)
Coal reduction can produce Fe, Cu, Zn, Cd, Ge, Sn, Pb and other metals that do not form strong carbides (compounds with carbon).
Hydrogen or active metals can be used as a reducing agent:
1) MoO 3 + ZN 2 \u003d Mo + ZN 2 O (hydrothermy)
The advantages of this method include obtaining a very pure metal.
2) TiO 2 + 2Mg \u003d Ti + 2MgO (magnesium)
ZMnO 2 + 4Al \u003d ZMn + 2Al 2 O 3 (aluminothermy)
Most often, aluminum is used in metallothermy, the heat of oxide formation
which is very large (2A1 + 1.5 O 2 \u003d Al 2 O 3 + 1676 kJ / mol). The electrochemical series of voltages of metals cannot be used to determine the possibility of reactions of metal reduction from their oxides. The possibility of this process can be approximately established based on the calculation of the thermal effect of the reaction (Q), knowing the values of the heats of formation of oxides:
Q \u003d Σ Q 1 - Σ Q 2,
where Q 1 is the heat of formation of the product, Q 2 is the heat of formation of the initial substance.
Blast furnace process (iron production):
C + O 2 \u003d CO 2, CO 2 + C ↔ 2CO
3Fe 2 O 3 + CO \u003d 2 (Fe 2 Fe 3 2) O 4 + CO 2
(Fe 2 Fe 3 2) O 4 + CO \u003d 3FeO + CO 2
FeO + CO \u003d Fe + CO 2
(cast iron contains up to 6.67% carbon in the form of grains of graphite and cementite Fe 3 C);

steel smelting(0.2-2.06% carbon) is carried out in special furnaces (converter, open-hearth, electric), differing in the method of heating. Blowing oxygen-enriched air burns out excess carbon from cast iron, as well as sulfur, phosphorus and silicon in the form of oxides. In this case, oxides are either captured in the form of exhaust gases (CO 2, SO 2), or are bound into an easily separated slag - a mixture of Ca 3 (PO 4) 2 and CaSiO 3. To obtain special steels, alloying additives of other metals are introduced into the furnace.
Hydrometallurgy
Hydrometallurgy is the recovery of metals from their salts in solution.
The process takes place in two stages: 1) a natural compound is dissolved in a suitable reagent to obtain a solution of a salt of this metal; 2) from the resulting solution, this metal is displaced by a more active one or restored by electrolysis. For example, to obtain copper from an ore containing copper oxide CuO, it is treated with dilute sulfuric acid:
CuO + H 2 SO 4 \u003d CuSO 4 + H 2
The copper is then either removed from the salt solution by electrolysis or displaced from the sulfate with iron:
CuSO 4 . + Fe \u003d Cu + FeSO 4
Thus, silver, zinc, molybdenum, gold, uranium are obtained.
Electrometallurgy
Electrometallurgy— recovery of metals in the process of electrolysis of solutions or melts of their compounds.
This method produces aluminum, alkali metals, alkaline earth metals. In this case, melts of oxides, hydroxides or chlorides are subjected to electrolysis.
Examples:  a) NaCl (melt electrolysis) → 2Na + Cl 2
a) NaCl (melt electrolysis) → 2Na + Cl 2
b) CaCl 2 (melt electrolysis) → Ca + Cl
c) 2Al 2 O 3 (melt electrolysis) → 2Al + 3O 2
d) 2Cr 2 (SO 4) + 6H 2 O (electrolysis) → 4Cr ↓ + 3O 2 + 6H 2 SO 4
e) 2MnSO 4 + 2H 2 O (electrolysis) → 2Mn↓ + O 2 + 2H 2 SO 4
f) FeCl 2 (solution electrolysis) → Fe ↓ + Cl 2

Metals are found in nature mainly in the form of compounds. Only metals with low chemical activity (noble metals) are found in nature in a free state (platinum metals, gold, copper, silver, mercury). Of the structural metals, only iron, aluminum, and magnesium are found in nature in the form of compounds in sufficient quantities. They form powerful deposits of deposits of relatively rich ores. This makes it easier to harvest them on a large scale.
Since the metals in the compounds are in an oxidized state (have a positive oxidation state), getting them in a free state is reduced to a reduction process:
This process can be carried out chemically or electrochemically.
In chemical reduction, coal or carbon monoxide (II), as well as hydrogen, active metals, and silicon are most often used as a reducing agent. With the help of carbon monoxide (II), iron is obtained (in the blast furnace process), many non-ferrous metals (tin, lead, zinc, etc.):
Hydrogen reduction is used, for example, to produce tungsten from tungsten(VI) oxide:
The use of hydrogen as a reducing agent ensures the highest purity of the resulting metal. Hydrogen is used to produce very pure iron, copper, nickel and other metals.
The method of obtaining metals, in which metals are used as a reducing agent, is called metallothermic . In this method, active metals are used as a reducing agent. Examples of metallothermic reactions:
aluminothermy:
magnesiumthermy:
Metal-thermal experiments for obtaining metals were first carried out by the Russian scientist N. N. Beketov in the 19th century.
Metals are most often obtained by the reduction of their oxides, which in turn are isolated from the corresponding natural ore. If the original ore is sulfide minerals, then the latter are subjected to oxidative roasting, for example:
Electrochemical production of metals is carried out during the electrolysis of melts of the corresponding compounds. In this way, the most active metals, alkali and alkaline earth metals, aluminum, and magnesium are obtained.
Electrochemical reduction is also used for refining (purification) of "raw" metals (copper, nickel, zinc, etc.) obtained by other methods. In electrolytic refining, a “rough” (with impurities) metal is used as an anode, and a solution of compounds of this metal is used as an electrolyte.
Methods for obtaining metals, carried out at high temperatures, are called pyrometallurgical (in Greek pyr - fire). Many of these methods have been known since ancient times. At the turn of the XIX-XX centuries. begin to develop hydrometallurgical methods of obtaining metals (in Greek hydor-water). With these methods, the ore components are transferred into an aqueous solution and then the metal is isolated by electrolytic or chemical reduction. So get, for example, copper. Copper ore containing copper (II) oxide CuO is treated with dilute sulfuric acid:
To reduce copper, the resulting solution of copper (II) sulfate is either subjected to electrolysis, or the solution is treated with iron powder.
The hydrometallurgical method has a great future, as it makes it possible to obtain a product without extracting the ore from the ground. (Compare the advantages of the hydrometallurgical method of obtaining metals with underground coal gasification.)
Metals in nature can be in the form of minerals, rocks, aqueous solutions. Only a few (Au, Pt, partly Ag, Cu, Hg) occur in the free state.
Mineral- an individual substance with a specific crystalline structure (for example, chalk, marble is calcium carbonate). Rock - a mixture of minerals. A rock that contains a significant amount of metals is called ore. Aqueous solutions – ocean and sea water; mineral water (in solutions, metals are in the form of salts).
Metallurgy is a science that studies and develops industrial methods for obtaining metals from ores.
Before receiving metals, the ore is enriched (concentrated), i.e., separated from the waste rock.
There are various ways to enrich ores. The most commonly used flotation, gravity and magnetic methods.
For example, the content of copper in exploited ores usually does not exceed 1%, so preliminary enrichment is necessary. It is achieved by using the method of flotation of ores, based on the different adsorption properties of the surfaces of the particles of sulphurous metals and the surrounding waste rock of the silicate type. If, in water containing a small admixture of a low-polarity organic substance (for example, pine oil), we shake up the powder of finely ground copper ore and blow air through the entire system, then the particles of copper sulfide, together with air bubbles, will rise up and flow over the edge of the vessel in the form of foam, and silicate particles will settle to the bottom. This is the basis of the flotation enrichment method, with the help of which more than 100 million tons of sulfur ores of various metals are processed annually. Enriched ore - concentrate - usually contains from 20 to 30% copper. With the help of selective (selective) flotation, it is possible not only to separate the ore from the waste rock, but also to separate the individual minerals of polymetallic ores.
Metallurgical processes are divided into pyrometallurgical and hydrometallurgical.
Pyrometallurgy– reduction of metals from their compounds (oxides, sulfides, etc.) under anhydrous conditions at high temperatures.
When processing sulfide ores, sulfides are first converted into oxides by roasting, and then the oxides are reduced with coal or CO:
ZnS + 3O 2 \u003d 2 ZnO + 2SO 2; 2PbS + 3O 2 \u003d 2 PbO + 2SO 2;
ZnO + C = Zn + CO; PbO + C = Pb + CO
The pyrometallurgical method produces, for example, cast iron and steel.
However, not all metals can be obtained by reducing their oxides with carbon or CO, so stronger reducing agents are used: hydrogen, magnesium, aluminum, silicon. For example, metals such as chromium, molybdenum, iron are aluminothermy :
3Fe 3 O 4 + 8Al \u003d 9Fe + 4Al 2 O 3.
Hydrometallurgy - extraction of metals from ores using aqueous solutions of certain reagents.
For example, an ore containing a basic salt (CuOH) 2 CO 3 is treated with a sulfuric acid solution:
(CuOH) 2 CO 3 + 2H 2 SO 4 \u003d 2CuSO 4 + 3H 2 O + CO 2.
From the resulting sulfate solution, copper is isolated either by electrolysis or by the action of metallic iron:
Fe + CuSO 4 \u003d Cu + FeSO 4.
The displacement of one metal by another from a solution of its salt is called in technology cementation.
Copper, zinc, cadmium, nickel, cobalt, manganese and other metals are obtained electrolysis salt solutions. The discharge of metal ions from solutions occurs at the cathode:
Cu+2+2 e -= Cu 0 .
These processes use insoluble anodes, which usually release oxygen:
2H2O-4 e -→ O 2 + 4H + .
Active metals (alkaline and alkaline earth) are obtained by electrolysis of melts, since these metals are soluble in water:
(cathode, -): Mg +2 + 2 e -= Mg 0 ; (anode, +): 2Cl – – 2 e -= Cl 2 0 .
Methods for cleaning metals
The properties of metals depend on the content of impurities in them. For example, titanium has not been used for a long time because of the fragility due to the presence of impurities. After the development of purification methods, the use of titanium has increased dramatically. Of particular importance is the purity of materials in electronic, computer technology and nuclear power.
Refining- the process of cleaning metals, based on the difference in the physical and chemical properties of the metal and impurities.
All methods of cleaning metals can be divided into chemical and physico-chemical.
Chemical Methods purifications consist in the interaction of metals with various reagents that form precipitates or gaseous products with base metals or impurities. To obtain high-purity nickel, iron, titanium, thermal decomposition of volatile metal compounds is used (carboxylic process, iodide process).
Consider, for example, the production of zirconium. In a closed system are iodine vapor and raw zirconium. The temperature in the reaction vessel is 300 ºС. At this temperature, volatile zirconium tetraiodide is formed on the surface of zirconium:
Zr (tv) + 2I 2 (g) ↔ ZrI 4 (g).
The reaction vessel contains a tungsten filament heated to 1500 ºС. Due to the high reversibility of this reaction, zirconium iodide is deposited on the tungsten filament and decomposed to form zirconium.
Physical and chemical methods include electrochemical, distillation, crystallization and other purification methods.
Electrolysis is widely used in the metallurgy of light and non-ferrous metals. This method is used to purify many metals: copper, silver, gold, lead, tin, etc.
Consider, for example, the refining of black nickel, which contains impurities of zinc and copper and serves as an anode in an electrolytic cell:
E 0 Zn 2+ / Zn = - 0.76 V; E 0 Cu 2+ / Cu = .34 V; E 0 Ni 2+ / Ni = - 0.25 V.
At the anode, the metal with the most negative potential dissolves first. Because
E 0 Zn 2+ / Zn< E 0 Ni 2+ / Ni< E 0 Cu 2+ / Cu ,
then zinc dissolves first, and then the base metal - nickel:
Zn-2 e-→ Zn 2 + , Ni - 2 e– → Ni 2 + .
The copper impurity, which has a more positive potential, does not dissolve and precipitates (sludge) in the form of metal particles. The solution will contain Zn 2+ and Ni 2+ ions. On the cathode, the metal with the most positive potential, i.e., nickel, is deposited first. Thus, as a result of refining, nickel is deposited on the cathode, copper precipitates into the sludge, and zinc goes into solution.
Electrolysis of melts of compounds produces aluminum, magnesium, sodium, lithium, beryllium, calcium, as well as alloys of some metals. The largest electrolytic process in the chemical industry is the electrolysis of a NaCl solution with the production of gaseous chlorine at the anode, hydrogen at the cathode, and an alkali solution in the cathode space. In addition, electrolysis produces fluorine from a melt mixture of HF and NaF, hydrogen and oxygen from water (to reduce ohmic losses, electrolysis is carried out in a NaOH solution), manganese dioxide from a MnSO 4 solution, etc.
Widely used zone melting , which consists in the fact that the heating zone and, accordingly, the zone of molten metal slowly move along the ingot (rod). Some impurities are concentrated in the melt and are collected at the end of the ingot, others - at the beginning of the ingot. After multiple runs, the initial and final parts of the ingot are cut off, leaving the cleaned middle part of the metal.
metal alloys
Alloy – it is a system with metallic properties, consisting of two or more metals (one component may be a non-metal).
Questions of the chemical interaction of metals with each other, as well as with non-metals, if the products of their interaction retain metallic properties, are studied by one of the sections of inorganic chemistry - metal chemistry .
If you arrange the metals in order of increasing their chemical interaction with each other, you get the following series:
– the components do not interact with each other either in the liquid or in the solid state;
- the components mutually dissolve in the liquid state, and form a eutectic in the solid state (mechanical mixture);
– components form with each other liquid and solid solutions of any composition (systems with unlimited solubility);
- the components form one or more metal compounds with each other, called intermetallic (system with the formation of a chemical compound).
To study the physical properties of alloys, depending on their composition, physicochemical analysis is widely used. This makes it possible to detect and study the chemical changes occurring in the system.
Chemical transformations in the system can be judged by the nature of the change in various physical properties - melting and crystallization temperatures, vapor pressure, viscosity, density, hardness, magnetic properties, electrical conductivity of the system, depending on its composition. Of the various types of physicochemical analysis, the most commonly used thermal analysis . During the analysis, they build and study melting charts, which are a plot of the melting point of the system versus its composition.
To build a melting diagram, two pure substances are taken and mixtures of various compositions are prepared from them. Each mixture is melted and then slowly cooled, noting the temperature of the cooling alloy at regular intervals. In this way a cooling curve is obtained. On fig. 1. shows the cooling curves of a pure substance (1) and alloy ( 2 ). The transition of a pure substance from a liquid to a solid state is accompanied by the release of heat of crystallization, therefore, until the entire liquid crystallizes, the temperature remains constant (section bc, curve 1 ). Further, the cooling of the solid proceeds evenly.
When the melt (solution) is cooled, the cooling curve has a more complex form (Fig. 1, curve 2). In the simplest case of cooling a melt of two substances, at first, a uniform decrease in temperature occurs until crystals of one of the substances begin to separate from the solution. Since the crystallization temperature of the solution is lower than that of the pure solvent, the crystallization of one of the substances from the solution begins above the crystallization temperature of the solution. When crystals of one of the substances are isolated, the composition of the liquid melt changes, and its solidification temperature continuously decreases as it crystallizes. The heat released during crystallization somewhat slows down the course of cooling and therefore, starting from the point l on the curve 2, the steepness of the cooling curve line decreases. Finally, when the melt becomes saturated with respect to both substances , crystallization of both substances begins simultaneously. This corresponds to the appearance of a horizontal section on the cooling curve b`c`. When crystallization ends, a further drop in temperature is observed.
Based on the cooling curves of mixtures of different compositions, a melting diagram is constructed. Let's consider the most typical of them.
Similar information.
Natural metal compounds
Metals can occur in nature either as a simple substance or as a complex substance.
Metals occur naturally in three forms:
1. Active - in the form of salts (sulfates, nitrates, chlorides, carbonates)
2. Medium activity - in the form of oxides, sulfides ( Fe 3 O 4 , FeS 2 )
3. Noble - in free form ( Au, Pt, Ag)
Most often, metals in nature are found in the form of salts of inorganic acids or oxides:
- chlorides - sylvinite KCl NaCl, rock salt NaCl;
- nitrates - Chilean saltpeter NaNO 3;
- sulfates - Glauber's salt Na 2 SO 4 10 H 2 O, gypsum CaSO 4 2H 2 O;
- carbonates - chalk, marble, limestone CaCO 3, magnesite MgCO 3, dolomite CaCO 3 MgCO 3;
- sulfides - sulfur pyrite FeS 2, cinnabar HgS, zinc blende ZnS;
- phosphates - phosphorites, apatites Ca 3 (PO 4) 2;
- oxides - magnetic iron ore Fe 3 O 4, red iron ore Fe 2 O 3, brown iron ore Fe 2 O 3 H 2 O.
Even in the middle of the II millennium BC. e. In Egypt, the production of iron from iron ores was mastered. This marked the beginning of the Iron Age in the history of mankind, which replaced the Stone and Bronze Ages. On the territory of our country, the beginning of the Iron Age is attributed to the turn of the II and I millennia BC. e.
Minerals and rocks containing metals and their compounds and suitable for the industrial production of metals are called ores.
The branch of industry that is engaged in obtaining metals from ores is called metallurgy. The science of industrial methods for obtaining metals from ores is also called.
Metallurgyis the science of industrial methods for producing metals.
Obtaining metals
Most metals are found in nature in the composition of compounds in which the metals are in a positive oxidation state, which means that in order to obtain them in the form of a simple substance, it is necessary to carry out a reduction process.
Me + n + ne - → Me 0
I. P pyrometallurgical method
This is the recovery of metals from their ores at high temperatures with the help of non-metallic reducing agents - coke, carbon monoxide (II), hydrogen; metal - aluminum, magnesium, calcium and other metals.
1. Obtaining copper from oxide using hydrogen - Hydrothermy :
Cu +2 O + H 2 \u003d Cu 0 + H 2 O
2. Obtaining iron from oxide using aluminum - Aluminothermy:
Fe +3 2 O 3 +2 Al \u003d 2 Fe 0 + Al 2 O 3
To obtain iron in industry, iron ore is subjected to magnetic enrichment:
3Fe 2 O 3 + H 2 \u003d 2Fe 3 O 4 + H 2 O or 3Fe 2 O 3 + CO \u003d 2Fe 3 O 4 + CO 2, and then the reduction process takes place in a vertical furnace:
Fe 3 O 4 + 4H 2 \u003d 3Fe + 4H 2 O
Fe 3 O 4 + 4CO \u003d 3Fe + 4CO 2
II. Hydrometallurgical method
The method is based on the dissolution of a natural compound in order to obtain a solution of a salt of this metal and the displacement of this metal by a more active one.
For example, the ore contains copper oxide and is dissolved in sulfuric acid:
1 stage - CuO + H 2 SO 4 \u003d CuSO 4 + H 2 O,
Stage 2 - carry out a substitution reaction with a more active metal
CuSO 4 + Fe \u003d FeSO 4 + Cu.
III. Electrometallurgical method
These are methods of obtaining metals using electric current (electrolysis).
This method produces aluminum, alkali metals, alkaline earth metals.
In this case, melts of oxides, hydroxides or chlorides are subjected to electrolysis:
2NaCl electric current → 2Na + Cl 2
2Al 2 O 3 electric current → 4Al + 3O 2
IV. Thermal decomposition of compounds
For example, getting iron:
Iron interacts with carbon monoxide (II) at elevated pressure and a temperature of 100-200 0, forming pentacarbonyl:
Fe + 5CO = Fe (CO) 5
Iron pentacarbonyl is a liquid that can be easily separated from impurities by distillation. At a temperature of about 250 0, carbonyl decomposes, forming iron powder:
Fe (CO) 5 \u003d Fe + 5CO
If the resulting powder is subjected to sintering in a vacuum or in a hydrogen atmosphere, then a metal containing 99.98–99.999% iron will be obtained.
Reactions underlying the production of metals
|
1. Recovery of metals from oxides with coal or carbon monoxide M x O y + C = CO 2 + Me or M x O y + CO = CO 2 + Me |
|
2. Sulfide roasting followed by reduction Stage 1 - M x S y + O 2 \u003d M x O y + SO 2 Stage 2 -M x O y + C \u003d CO 2 + Me or M x O y + CO \u003d CO 2 + Me |
|
3. Aluminothermy (recovery with a more active metal) M x O y + Al \u003d Al 2 O 3 + Me |
|
4. Hydrogen thermal M x O y + H 2 \u003d H 2 O + Me |
Thus, we got acquainted with natural metal compounds and methods for isolating metal from them as a simple substance.
- How to take B vitamins
- Reactions of glucose by alcohol groups
- What is the difference between ethyl alcohol and methyl alcohol
- What is the need for blood
- C6h12o6 chemical properties
- Methods for obtaining metals
- How to remove itching, redness and swelling?
- Methods for examining the intestines without colonoscopy
- Drugs. Signs of use. Help for drug addicts. The main signs of drug addiction The three main signs of drug addiction
- The main symptoms of the disease
- How is the treatment of psychoses and neuroses of varying severity?
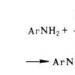
- Amines - concept, properties, application
- What can be transmitted through saliva
- Chemical properties of proteins
- Pills for fever
- How to protect the liver when using drugs?
- Vitamin A deficiency: reasons for what to do
- Which is better: Theraflu or Coldrex?
- Ulcerative colitis of the intestine - symptoms, treatment, causes
- Lichen sclerosus: causes, symptoms and treatment Lichen disease