Proteins are synthesized as a result of a reaction. Chemical properties of proteins. Protein isolation and purification methods
4. Classification of proteins
Proteins and their main features
Proteins or proteins (which in Greek means "first" or "most important") quantitatively predominate over all macromolecules present in a living cell, and make up more than half of the dry weight of most organisms. The concept of proteins as a class of compounds was formed in the 17th-19th centuries. During this period, substances with similar properties were isolated from various objects of the living world (seeds and juices of plants, muscles, blood, milk): they formed viscous solutions, coagulated when heated, the smell of burnt wool was felt during combustion and ammonia was released. Since all these properties were previously known for egg white, the new class of compounds was called proteins. After the appearance at the beginning of the XIX century. More advanced methods of analysis of substances determined the elemental composition of proteins. They found C, H, O, N, S. By the end of the 19th century. More than 10 amino acids have been isolated from proteins. Based on the results of studying the products of protein hydrolysis, the German chemist E. Fischer (1852-1919) suggested that proteins are built from amino acids.
As a result of Fisher's work, it became clear that proteins are linear polymers of a-amino acids connected to each other by an amide (peptide) bond, and the whole variety of representatives of this class of compounds could be explained by differences in the amino acid composition and the order of alternation of different amino acids in the polymer chain.
The first studies of proteins were carried out with complex protein mixtures, for example: with blood serum, egg white, extracts of plant and animal tissues. Later, methods for isolating and purifying proteins were developed, such as precipitation, dialysis, chromatography on cellulose and other hydrophilic ion exchangers, gel filtration, and electrophoresis. We will consider these methods in more detail in the laboratory work and seminar.
At the present stage, the main areas of study of proteins are the following:
¨ study of the spatial structure of individual proteins;
¨ study of the biological functions of different proteins;
¨ study of the mechanisms of functioning of individual proteins (at the level of individual atoms, atomic groups of a protein molecule).
All these stages are interrelated, because one of the main tasks of biochemistry is precisely to understand how the amino acid sequences of different proteins enable them to perform various functions.
Biological functions of proteins
Enzymes - they are biological catalysts, the most diverse and numerous class of proteins. Almost all chemical reactions involving organic biomolecules present in the cell are catalyzed by enzymes. To date, more than 2000 different enzymes have been discovered.
Transport proteins- Transport proteins in blood plasma bind and carry specific molecules or ions from one organ to another. For example, hemoglobin, contained in erythrocytes, when passing through the lungs, it binds oxygen and delivers it to peripheral tissues, where oxygen is released. The blood plasma contains lipoproteins that transport lipids from the liver to other organs. In cell membranes, there is another type of cellular transport proteins that can bind certain molecules (eg, glucose) and transport them through the membrane into the cell.
Dietary and storage proteins. The best-known examples of such proteins are wheat, corn, and rice seed proteins. Dietary proteins are egg albumin- the main component of egg white, casein is the main protein in milk.
Contractile and motor proteins.Actin And myosin- proteins that function in the contractile system of skeletal muscle, as well as in many non-muscle tissues.
Structural proteins.Collagen- the main component of cartilage and tendons. This protein has a very high tensile strength. Bundles contain elastin- a structural protein capable of stretching in two dimensions. Hair, nails are composed almost exclusively of durable insoluble protein - keratin. The main component of silk threads and cobwebs is the protein fibroin.
protective proteins. Immunoglobulins or antibodies are specialized cells produced in lymphocytes. They have the ability to recognize viruses or foreign molecules that have entered the body of bacteria, and then launch a system to neutralize them. fibrinogen And thrombin- proteins involved in the process of blood clotting, they protect the body from blood loss when the vascular system is damaged.
regulatory proteins. Some proteins are involved in the regulation of cellular activity. These include many hormones such as insulin (regulates glucose metabolism).
Protein classification
By solubility
Albumins. Soluble in water and saline solutions.
Globulins. Slightly soluble in water, but highly soluble in saline solutions.
Prolamins. Soluble in 70-80% ethanol, insoluble in water and absolute alcohol. Rich in arginine.
Histones. Soluble in saline solutions.
Scleroproteins. Insoluble in water and saline solutions. The content of glycine, alanine, proline is increased.
The shape of the molecules
Based on the ratio of the axes (longitudinal and transverse), two large classes of proteins can be distinguished. At globular proteins the ratio is less than 10 and in most cases does not exceed 3-4. They are characterized by compact packing of polypeptide chains. Examples of globular proteins: many enzymes, insulin, globulin, plasma proteins, hemoglobin.
fibrillar proteins, in which the ratio of the axes exceeds 10, consist of bundles of polypeptide chains spirally wound on top of each other and interconnected by transverse covalent or hydrogen bonds (keratin, myosin, collagen, fibrin).
Physical properties of proteins
On the physical properties of proteins such as ionization,hydration, solubility various methods for isolating and purifying proteins are based.
Since proteins contain ionogenic, i.e. ionizable amino acid residues (arginine, lysine, glutamic acid, etc.), therefore, they are polyelectrolytes. With acidification, the degree of ionization of anionic groups decreases, while that of cationic groups increases; with alkalization, the opposite pattern is observed. At a certain pH, the number of negatively and positively charged particles becomes the same, this state is called isoelectric(the total charge of the molecule is zero). The pH value at which a protein is in an isoelectric state is called isoelectric point and denote pI. One of the methods for their separation is based on the different ionization of proteins at a certain pH value - the method electrophoresis.
Polar groups of proteins (ionic and nonionic) are able to interact with water and hydrate. The amount of water associated with protein reaches 30-50 g per 100 g of protein. There are more hydrophilic groups on the surface of the protein. Solubility depends on the number of hydrophilic groups in the protein, on the size and shape of the molecules, and on the magnitude of the total charge. The combination of all these physical properties of the protein makes it possible to use the method molecular sieves or gel filtration to separate proteins. Method dialysis is used to purify proteins from low molecular weight impurities and is based on the large size of protein molecules.
The solubility of proteins also depends on the presence of other solutes, such as neutral salts. At high concentrations of neutral salts, proteins precipitate, and for precipitation ( salting out) different proteins require different concentrations of salt. This is due to the fact that charged protein molecules adsorb ions of opposite charge. As a result, the particles lose their charges and electrostatic repulsion, resulting in protein precipitation. The salting out method can be used to fractionate proteins.
Primary structure of proteins
Primary structure of a protein name the composition and sequence of amino acid residues in a protein molecule. Amino acids in a protein are linked by peptide bonds.
All molecules of a given individual protein are identical in amino acid composition, sequence of amino acid residues, and length of the polypeptide chain. Establishing the sequence of the amino acid sequence of proteins is a time-consuming task. We will discuss this topic in more detail at the seminar. Insulin was the first protein to have its amino acid sequence determined. Bovine insulin has a molar mass of about 5700. Its molecule consists of two polypeptide chains: an A chain containing 21 a.a., and a B chain containing 30 a.k., these two chains are connected by two disulfide (-S-S-) connections. Even small changes in the primary structure can significantly change the properties of a protein. The disease sickle cell anemia is the result of a change in just 1 amino acid in the b-chain of hemoglobin (Glu ® Val).
Species specificity of the primary structure
When studying amino acid sequences homologous proteins isolated from different species, several important conclusions were made. Homologous proteins are those proteins that perform the same functions in different species. An example is hemoglobin: in all vertebrates, it performs the same function associated with the transport of oxygen. Homologous proteins of different species usually have polypeptide chains of the same or nearly the same length. In the amino acid sequences of homologous proteins, the same amino acids are always found in many positions - they are called invariant residues. At the same time, significant differences are observed in other positions of proteins: in these positions, amino acids vary from species to species; such amino acid residues are called variable. The whole set of similar features in the amino acid sequences of homologous proteins is combined into the concept sequence homology. The presence of such homology suggests that the animals from which the homologous proteins were isolated share a common evolutionary origin. An interesting example is a complex protein - cytochrome c- mitochondrial protein involved as an electron carrier in the processes of biological oxidation. M » 12500, contains » 100 a.a. A.K. were installed. sequences for 60 species. 27 a.c. - are the same, which indicates that all these residues play an important role in determining the biological activity of cytochrome c. The second important conclusion drawn from the analysis of amino acid sequences is that the number of residues by which cytochromes differ from any two species is proportional to the phylogenetic difference between these species. For example, the molecules of cytochrome c from a horse and yeast differ by 48 a.a., in duck and chicken - by 2 a.a., in chicken and turkey they do not differ. Information on the number of differences in the amino acid sequences of homologous proteins from different species is used to build evolutionary maps that reflect the successive stages of the emergence and development of various animal and plant species in the evolutionary process.
Secondary structure of proteins
- this is the packing of a protein molecule in space without taking into account the influence of side substituents. There are two types of secondary structure: a-helix and b-structure (folded layer). Let us dwell in more detail on the consideration of each type of secondary structure.
a-Spiral is a right helix with the same pitch equal to 3.6 amino acid residues. The a-helix is stabilized by intramolecular hydrogen bonds that occur between the hydrogen atoms of one peptide bond and the oxygen atoms of the fourth peptide bond.
![]()
The side substituents are located perpendicular to the plane of the a-helix.
 |
That. the properties of a given protein are determined by the properties of the side groups of amino acid residues that are part of a particular protein. If the side substituents are hydrophobic, then the protein having the a-helix structure is also hydrophobic. An example of such a protein is the keratin protein that makes up hair.
As a result, it turns out that the a-helix is permeated with hydrogen bonds and is a very stable structure. In the formation of such a spiral, two tendencies work:
¨ the molecule tends to a minimum of energy, i.e. to the formation of the largest number of hydrogen bonds;
¨ due to the rigidity of the peptide bond, only the first and fourth peptide bonds can approach each other in space.
IN folded layer peptide chains are arranged parallel to each other, forming a figure similar to a sheet folded like an accordion. There can be a large number of peptide chains interacting with each other by hydrogen bonds. The chains are arranged antiparallel.
 |
The more peptide chains that make up the folded layer, the stronger the protein molecule.
Let us compare the properties of the protein materials of wool and silk and explain the difference in the properties of these materials in terms of the structure of the proteins of which they are composed.
Keratin - wool protein - has an a-helix secondary structure. Woolen thread is not as strong as silk, it stretches easily when wet. This property is explained by the fact that when a load is applied, the hydrogen bonds break and the helix stretches.
Fibroin - silk protein - has a secondary b-structure. The silk thread does not stretch and is very tear-resistant. This property is explained by the fact that in the folded layer many peptide chains interact with each other by hydrogen bonds, which makes this structure very strong.
Amino acids differ in their ability to participate in the formation of a-helices and b-structures. Glycine, aspargine, tyrosine are rarely found in a-helices. Proline destabilizes the a-helical structure. Explain why? The composition of b-structures includes glycine, almost no proline, glutamic acid, aspargine, histidine, lysine, serine.
The structure of one protein may contain sections of b-structures, a-helices, and irregular sections. In irregular regions, the peptide chain can relatively easily bend and change conformation, while the helix and the folded layer are fairly rigid structures. The content of b-structures and a-helices in different proteins is not the same.
Tertiary structure of proteins
determined by the interaction of the side substituents of the peptide chain. For fibrillar proteins, it is difficult to identify general patterns in the formation of tertiary structures. As for globular proteins, such regularities exist, and we will consider them. The tertiary structure of globular proteins is formed by additional folding of the peptide chain containing b-structures, a-helices and irregular regions, so that the hydrophilic side groups of amino acid residues are on the surface of the globule, and the hydrophobic side groups are hidden deep into the globule, sometimes forming a hydrophobic pocket.
Forces that stabilize the tertiary structure of a protein.
Electrostatic interaction between differently charged groups, the extreme case is ionic interactions.
Hydrogen bonds arising between the side groups of the polypeptide chain.
Hydrophobic interactions.
covalent interactions(formation of a disulfide bond between two cysteine residues to form cystine). The formation of disulfide bonds leads to the fact that the remote regions of the polypeptide molecule approach each other and are fixed. Disulfide bonds are broken by reducing agents. This property is used to perm hair, which is almost entirely a keratin protein, riddled with disulfide bonds.
The nature of the spatial packing is determined by the amino acid composition and the alternation of amino acids in the polypeptide chain (primary structure). Therefore, each protein has only one spatial structure corresponding to its primary structure. Small changes in the conformation of protein molecules occur when interacting with other molecules. These changes sometimes play a huge role in the functioning of protein molecules. So, when an oxygen molecule is attached to hemoglobin, the conformation of the protein changes somewhat, which leads to the effect of cooperative interaction when the remaining three oxygen molecules are attached. Such a change in the conformation in underlies the theory of inducing correspondence in explaining the group specificity of some enzymes.
In addition to the covalent disulfide bond, all other bonds stabilizing the tertiary structure are inherently weak and easily destroyed. When a large number of bonds stabilizing the spatial structure of a protein molecule are broken, the ordered conformation, unique for each protein, is broken, and the biological activity of the protein is often lost. This change in spatial structure is called denaturation.
Protein function inhibitors
Considering that different ligands differ in Kb, it is always possible to choose a substance similar in structure to the natural ligand, but having a higher Kb value with a given protein. For example, CO has a K St 100 times greater than O 2 with hemoglobin, so 0.1% CO in the air is enough to block a large number of hemoglobin molecules. Many medicines work on the same principle. For example, dithylin.

Acetylcholine is a mediator for the transmission of nerve impulses to the muscle. Ditilin blocks the receptor protein to which acetylcholine binds and creates the effect of paralysis.
9. Connection between the structure of proteins and their functions on the example of hemoglobin and myoglobin
Transport of carbon dioxide
Hemoglobin not only carries oxygen from the lungs to peripheral tissues, but also accelerates the transport of CO 2 from tissues to the lungs. Hemoglobin binds CO 2 immediately after the release of oxygen (» 15% of total CO 2). In erythrocytes, an enzymatic process of formation of carbonic acid from CO 2 coming from tissues occurs: CO 2 + H 2 O \u003d H 2 CO 3. Carbonic acid quickly dissociates into HCO 3 - and H +. To prevent a dangerous increase in acidity, there must be a buffer system capable of absorbing excess protons. Hemoglobin binds two protons for every four oxygen molecules released and determines the buffering capacity of the blood. In the lungs, the process is reversed. The released protons bind to the bicarbonate ion to form carbonic acid, which, under the action of the enzyme, is converted into CO 2 and water, CO 2 is exhaled. Thus, the binding of O 2 is closely associated with the exhalation of CO 2 . This reversible phenomenon is known as Bohr effect. Myoglobin does not exhibit the Bohr effect.
Isofunctional proteins
A protein that performs a specific function in a cell can be represented by several forms - isofunctional proteins, or isoenzymes. Although such proteins perform the same function, they differ in the binding constant, which leads to some differences in functional terms. For example, several forms of hemoglobin were found in human erythrocytes: HbA (96%), HbF (2%), HbA 2 (2%). All hemoglobins are tetramers built from protomers a, b, g, d (HbA - a 2 b 2, HbF - a 2 g 2, HbA 2 - a 2 d 2). All protomers are similar to each other in the primary structure, and a very large similarity is observed in the secondary and tertiary structures. All forms of hemoglobin are designed to carry oxygen to tissue cells, but HbF, for example, has a greater affinity for oxygen than HbA. HbF is characteristic of the embryonic stage of human development. It is able to take oxygen from HbA, which ensures a normal supply of oxygen to the fetus.
Isoproteins are the result of having more than one structural gene in a species' gene pool.
PROTEINS: STRUCTURE, PROPERTIES AND FUNCTIONS
1. Proteins and their main features
2. Biological functions of proteins
3. Amino acid composition of proteins
4. Classification of proteins
5. Physical properties of proteins
6. Structural organization of protein molecules (primary, secondary, tertiary structures)
Proteins are biopolymers, the monomers of which are alpha-amino acid residues interconnected by peptide bonds. The amino acid sequence of each protein is strictly defined; in living organisms, it is encrypted by means of the genetic code, on the basis of which the biosynthesis of protein molecules takes place. 20 amino acids are involved in building proteins.
There are the following types of structure of protein molecules:
- Primary. It is an amino acid sequence in a linear chain.
- Secondary. This is a more compact stacking of polypeptide chains through the formation of hydrogen bonds between peptide groups. There are two variants of the secondary structure - alpha helix and beta folding.
- Tertiary. Represents the laying of a polypeptide chain into a globule. In this case, hydrogen, disulfide bonds are formed, and the stabilization of the molecule is also realized due to hydrophobic and ionic interactions of amino acid residues.
- Quaternary. A protein consists of several polypeptide chains that interact with each other through non-covalent bonds.
Thus, amino acids connected in a certain sequence form a polypeptide chain, the individual parts of which coil or form folds. Such elements of secondary structures form globules, forming the tertiary structure of the protein. Individual globules interact with each other, forming complex protein complexes with a quaternary structure.
Protein classification
There are several criteria by which protein compounds can be classified. The composition distinguishes between simple and complex proteins. Complex protein substances contain non-amino acid groups in their composition, the chemical nature of which may be different. Depending on this, there are:
- glycoproteins;
- lipoproteins;
- nucleoproteins;
- metalloproteins;
- phosphoproteins;
- chromoproteins.
There is also a classification according to the general type of structure:
- fibrillar;
- globular;
- membrane.
Proteins are called simple (one-component) proteins, consisting only of amino acid residues. Depending on the solubility, they are divided into the following groups:
Such a classification is not entirely accurate, because according to recent studies, many simple proteins are associated with a minimum number of non-protein compounds. So, some proteins contain pigments, carbohydrates, sometimes lipids, which makes them more like complex protein molecules.
Physico-chemical properties of protein
The physicochemical properties of proteins are determined by the composition and number of amino acid residues included in their molecules. The molecular weights of polypeptides vary greatly, from a few thousand to a million or more. The chemical properties of protein molecules are diverse, including amphotericity, solubility, and the ability to denature.
Amphoteric
Since proteins contain both acidic and basic amino acids, the molecule will always contain free acidic and free basic groups (COO- and NH3+, respectively). The charge is determined by the ratio of basic and acidic amino acid groups. For this reason, proteins are charged “+” if the pH decreases, and vice versa, “-” if the pH increases. In the case when the pH corresponds to the isoelectric point, the protein molecule will have zero charge. Amphotericity is important for the implementation of biological functions, one of which is maintaining the pH level in the blood.
Solubility
The classification of proteins according to the property of solubility has already been given above. The solubility of proteins in water is explained by two factors:
- charge and mutual repulsion of protein molecules;
- the formation of a hydration shell around the protein - water dipoles interact with charged groups on the outer part of the globule.
Denaturation
The physicochemical property of denaturation is a process of destruction of the secondary, tertiary structure of a protein molecule under the influence of a number of factors: temperature, the action of alcohols, salts of heavy metals, acids and other chemical agents.
Important! The primary structure is not destroyed during denaturation.
Chemical properties of proteins, qualitative reactions, reaction equations
The chemical properties of proteins can be considered using the reactions of their qualitative detection as an example. Qualitative reactions make it possible to determine the presence of a peptide group in a compound:
1. Xanthoprotein. When high concentrations of nitric acid act on the protein, a precipitate is formed, which, when heated, becomes yellow.
2. Biuret. Under the action of copper sulfate on a weakly alkaline protein solution, complex compounds are formed between copper ions and polypeptides, which is accompanied by staining the solution in a violet-blue color. The reaction is used in clinical practice to determine the concentration of protein in blood serum and other biological fluids.
Another important chemical property is the detection of sulfur in protein compounds. For this purpose, an alkaline protein solution is heated with lead salts. This gives a black precipitate containing lead sulfide.
The biological significance of protein
Due to their physical and chemical properties, proteins perform a large number of biological functions, which include:
- catalytic (enzyme proteins);
- transport (hemoglobin);
- structural (keratin, elastin);
- contractile (actin, myosin);
- protective (immunoglobulins);
- signal (receptor molecules);
- hormonal (insulin);
- energy.
Proteins are important for the human body, since they are involved in the formation of cells, provide muscle contraction in animals, and carry many chemical compounds together with blood serum. In addition, protein molecules are a source of essential amino acids and perform a protective function, participating in the production of antibodies and the formation of immunity.
Top 10 Little Known Protein Facts
- Proteins began to be studied since 1728, it was then that the Italian Jacopo Bartolomeo Beccari isolated protein from flour.
- Recombinant proteins are now widely used. They are synthesized by modifying the bacterial genome. In particular, insulin, growth factors and other protein compounds that are used in medicine are obtained in this way.
- Protein molecules have been found in Antarctic fish that prevent blood from freezing.
- The resilin protein is characterized by ideal elasticity and is the basis of the attachment points of insect wings.
- The body has unique chaperone proteins that are able to restore the correct native tertiary or quaternary structure of other protein compounds.
- In the nucleus of the cell there are histones - proteins that take part in the compaction of chromatin.
- The molecular nature of antibodies - special protective proteins (immunoglobulins) - began to be actively studied since 1937. Tiselius and Kabat used electrophoresis and proved that in immunized animals the gamma fraction was increased, and after the absorption of serum by the provoking antigen, the distribution of proteins by fractions returned to the picture of the intact animal.
- Egg white is a vivid example of the implementation of a reserve function by protein molecules.
- In the collagen molecule, every third amino acid residue is formed by glycine.
- In the composition of glycoproteins, 15-20% are carbohydrates, and in the composition of proteoglycans their share is 80-85%.
Conclusion
Proteins are the most complex compounds, without which it is difficult to imagine the vital activity of any organism. More than 5,000 protein molecules have been isolated, but each individual has its own set of proteins and this differs from other individuals of its species.
The most important chemical and physical properties of proteins updated: March 21, 2019 by: Scientific Articles.Ru
Squirrels- natural polypeptides with a huge molecular weight. They are part of all living organisms and perform various biological functions.
The structure of the protein.
Proteins have 4 levels of structure:
- primary structure of a protein- linear sequence of amino acids in the polypeptide chain, folded in space:
- protein secondary structure- conformation of the polypeptide chain, because twisting in space due to hydrogen bonds between NH And SO groups. There are 2 installation methods: α -spiral and β - structure.
- protein tertiary structure is a three-dimensional representation of a swirling α - spiral or β -structures in space:

This structure is formed by disulfide bridges -S-S- between cysteine residues. Oppositely charged ions participate in the formation of such a structure.
- quaternary protein structure formed by the interaction between different polypeptide chains:

Protein synthesis.
The synthesis is based on the solid-phase method, in which the first amino acid is fixed on a polymer carrier, and new amino acids are sequentially sutured to it. The polymer is then separated from the polypeptide chain.
The physical properties of the protein.
The physical properties of the protein are determined by the structure, so the proteins are divided into globular(soluble in water) and fibrillar(insoluble in water).
Chemical properties of proteins.
1. Protein denaturation(destruction of the secondary and tertiary structure with the preservation of the primary). An example of denaturation is the curdling of egg whites when eggs are boiled.
2. Protein hydrolysis- irreversible destruction of the primary structure in an acidic or alkaline solution with the formation of amino acids. This way you can determine the quantitative composition of proteins.
3. Qualitative reactions:
Biuret reaction- interaction of the peptide bond and salts of copper (II) in an alkaline solution. At the end of the reaction, the solution turns purple.
xantoprotein reaction- when reacted with nitric acid, a yellow color is observed.
The biological significance of protein.
1. Proteins are a building material; muscles, bones, and tissues are built from it.
2. Proteins - receptors. They transmit and receive signals from neighboring cells from the environment.
3. Proteins play an important role in the body's immune system.
4. Proteins perform transport functions and carry molecules or ions to the place of synthesis or accumulation. (Hemoglobin carries oxygen to tissues.)
5. Proteins - catalysts - enzymes. These are very powerful selective catalysts that speed up reactions millions of times.
There are a number of amino acids that cannot be synthesized in the body - irreplaceable, they are obtained only with food: tizine, phenylalanine, methinine, valine, leucine, tryptophan, isoleucine, threonine.
On the physical properties of proteins such as ionization,hydration, solubility various methods for isolating and purifying proteins are based.
Since proteins contain ionogenic, i.e. ionizable amino acid residues (arginine, lysine, glutamic acid, etc.), therefore, they are polyelectrolytes. With acidification, the degree of ionization of anionic groups decreases, while that of cationic groups increases; with alkalization, the opposite pattern is observed. At a certain pH, the number of negatively and positively charged particles becomes the same, this state is called isoelectric(the total charge of the molecule is zero). The pH value at which a protein is in an isoelectric state is called isoelectric point and denote pI. One of the methods for their separation is based on the different ionization of proteins at a certain pH value - the method electrophoresis.
Polar groups of proteins (ionic and nonionic) are able to interact with water and hydrate. The amount of water associated with protein reaches 30-50 g per 100 g of protein. There are more hydrophilic groups on the surface of the protein. Solubility depends on the number of hydrophilic groups in the protein, on the size and shape of the molecules, and on the magnitude of the total charge. The combination of all these physical properties of the protein makes it possible to use the method molecular sieves or gel filtration to separate proteins. Method dialysis is used to purify proteins from low molecular weight impurities and is based on the large size of protein molecules.
The solubility of proteins also depends on the presence of other solutes, such as neutral salts. At high concentrations of neutral salts, proteins precipitate, and for precipitation ( salting out) different proteins require different concentrations of salt. This is due to the fact that charged protein molecules adsorb ions of opposite charge. As a result, the particles lose their charges and electrostatic repulsion, resulting in protein precipitation. The salting out method can be used to fractionate proteins.
6. Structural organization of protein molecules
Proteins are very large molecules, the molar mass of proteins ranges from 6000 to 1 million grams / mol (see table).
Some proteins in their composition may have chemical groups of a non-protein nature. Such proteins are called complex or holoproteins. The non-amino acid part of proteins is called prosthetic group, the protein part - apoenzyme. Complex proteins are classified according to their prosthetic group. For example, lipoproteins are proteins containing in their composition a group - a lipid; metalloproteins contain metal ions; chromoproteins include a chromophore, a colored group of non-protein nature. A special case when the chromophore is heme. These proteins include hemoglobin and cytochromes. Prosthetic groups play an important role in the functioning of a complex protein.
Simple proteins can be classified according to their molecular shape and their ability to dissolve in water for globular And fibrillar. Globular proteins are globule-shaped and are generally water soluble. . Fibrillar proteins have the form of an elongated fiber - fibrils and are insoluble in water. Fibrillar proteins perform mainly supporting functions, providing tissue strength; globular proteins are more diverse in function.
6.1. Primary structure of proteins
Primary structure of a protein name the composition and sequence of amino acid residues in a protein molecule. Amino acids in a protein are linked by peptide bonds.
All molecules of a given individual protein are identical in amino acid composition, sequence of amino acid residues, and length of the polypeptide chain. Establishing the sequence of the amino acid sequence of proteins is a time-consuming task. We will discuss this topic in more detail at the seminar. Insulin was the first protein to have its amino acid sequence determined. Bovine insulin has a molar mass of about 5700. Its molecule consists of two polypeptide chains: an A chain containing 21 a.a., and a B chain containing 30 a.k., these two chains are connected by two disulfide (-S-S-) connections. Even small changes in the primary structure can significantly change the properties of a protein. The disease sickle cell anemia is the result of a change in just 1 amino acid in the b-chain of hemoglobin (Glu ® Val).
Species specificity of the primary structure
When studying amino acid sequences homologous proteins isolated from different species, several important conclusions were made. Homologous proteins are those proteins that perform the same functions in different species. An example is hemoglobin: in all vertebrates, it performs the same function associated with the transport of oxygen. Homologous proteins of different species usually have polypeptide chains of the same or nearly the same length. In the amino acid sequences of homologous proteins, the same amino acids are always found in many positions - they are called invariant residues. At the same time, significant differences are observed in other positions of proteins: in these positions, amino acids vary from species to species; such amino acid residues are called variable. The whole set of similar features in the amino acid sequences of homologous proteins is combined into the concept sequence homology. The presence of such homology suggests that the animals from which the homologous proteins were isolated share a common evolutionary origin. An interesting example is a complex protein - cytochrome c- mitochondrial protein involved as an electron carrier in the processes of biological oxidation. M » 12500, contains » 100 a.a. A.K. were installed. sequences for 60 species. 27 a.c. - are the same, which indicates that all these residues play an important role in determining the biological activity of cytochrome c. The second important conclusion drawn from the analysis of amino acid sequences is that the number of residues by which cytochromes differ from any two species is proportional to the phylogenetic difference between these species. For example, the molecules of cytochrome c from a horse and yeast differ by 48 a.a., in duck and chicken - by 2 a.a., in chicken and turkey they do not differ. Information on the number of differences in the amino acid sequences of homologous proteins from different species is used to build evolutionary maps that reflect the successive stages of the emergence and development of various animal and plant species in the evolutionary process.
Spatial arrangement of polypeptide chains (Conformation of peptide chains in proteins)
The term conformation is used to describe the spatial arrangement of substituent groups in an organic molecule that can freely change their position in space without breaking any bonds.
The peptide chain has considerable flexibility. As a result of intrachain interactions, it acquires a certain spatial structure (conformation). In proteins, two levels of spatial organization are distinguished for one polypeptide chain: the secondary and tertiary structures of the protein. For proteins containing several polypeptide chains, it is possible that the spatial arrangement of these chains relative to each other is considered - the quaternary structure of the protein.
Squirrels - These are biopolymers consisting of α-amino acid residues interconnected by peptide bonds (-CO-NH-). Proteins are part of the cells and tissues of all living organisms. Protein molecules contain 20 different amino acid residues.
protein structure
Proteins have an inexhaustible variety of structures.
Primary structure of a protein is the sequence of amino acid units in a linear polypeptide chain.
secondary structure- this is a spatial configuration of a protein molecule, resembling a helix, which is formed as a result of twisting the polypeptide chain due to hydrogen bonds between groups: CO and NH.
Tertiary structure- this is the spatial configuration that the polypeptide chain twisted into a spiral takes.
Quaternary structure are polymeric formations of several protein macromolecules.
Physical properties
The properties of proteins are very diverse, which they perform. Some proteins dissolve in water, forming, as a rule, colloidal solutions (for example, egg white); others dissolve in dilute salt solutions; others are insoluble (for example, proteins of integumentary tissues).
Chemical properties
Denaturation- destruction of the secondary, tertiary structure of the protein under the influence of various factors: temperature, the action of acids, salts of heavy metals, alcohols, etc.
During denaturation under the influence of external factors (temperature, mechanical action, the action of chemical agents and other factors), a change occurs in the secondary, tertiary and quaternary structures of the protein macromolecule, that is, its native spatial structure. The primary structure and, consequently, the chemical composition of the protein do not change. Physical properties change: solubility decreases, ability to hydrate, biological activity is lost. The shape of the protein macromolecule changes, aggregation occurs. At the same time, the activity of some groups increases, the effect of proteolytic enzymes on proteins is facilitated, and, consequently, it is more easily hydrolyzed.
In food technology, thermal denaturation of proteins is of particular practical importance, the degree of which depends on temperature, heating time, and humidity. This must be remembered when developing modes of heat treatment of food raw materials, semi-finished products, and sometimes finished products. Thermal denaturation processes play a special role in blanching plant materials, drying grain, baking bread, and obtaining pasta. Protein denaturation can also be caused by mechanical action (pressure, rubbing, shaking, ultrasound). The action of chemical reagents (acids, alkalis, alcohol, acetone) leads to the denaturation of proteins. All these techniques are widely used in food and biotechnology.
Qualitative reactions to proteins:
a) When burning protein - the smell of burnt feathers.
b) Protein + HNO 3 → yellow color
c) Protein solution + NaOH + CuSO 4 → violet color
Hydrolysis
Protein + H 2 O → a mixture of amino acids
Functions of proteins in nature:
catalytic (enzymes);
Regulatory (hormones);
Structural (wool keratin, silk fibroin, collagen);
motor (actin, myosin);
transport (hemoglobin);
Spare (casein, egg albumin);
protective (immunoglobulins), etc.
Hydration
The process of hydration means the binding of water by proteins, while they exhibit hydrophilic properties: they swell, their mass and volume increase. Protein swelling is accompanied by its partial dissolution. The hydrophilicity of individual proteins depends on their structure. The hydrophilic amide (–CO–NH–, peptide bond), amine (NH 2), and carboxyl (COOH) groups present in the composition and located on the surface of the protein macromolecule attract water molecules, strictly orienting them to the surface of the molecule. Surrounding the protein globules, the hydrate (water) shell prevents the stability of protein solutions. At the isoelectric point, proteins have the least ability to bind water; the hydration shell around the protein molecules is destroyed, so they combine to form large aggregates. Aggregation of protein molecules also occurs when they are dehydrated with some organic solvents, such as ethyl alcohol. This leads to the precipitation of proteins. When the pH of the medium changes, the protein macromolecule becomes charged, and its hydration capacity changes.
With limited swelling, concentrated protein solutions form complex systems called jelly. The jellies are not fluid, elastic, have plasticity, a certain mechanical strength, and are able to retain their shape. Globular proteins can be completely hydrated by dissolving in water (for example, milk proteins), forming solutions with a low concentration. The hydrophilic properties of proteins are of great importance in biology and the food industry. A very mobile jelly, built mainly of protein molecules, is the cytoplasm - the semi-liquid contents of the cell. Highly hydrated jelly is raw gluten isolated from wheat dough and contains up to 65% water. Hydrophilicity, the main quality of wheat grain, grain proteins and flour, plays an important role in the storage and processing of grain, in baking. The dough, which is obtained in the bakery industry, is a protein swollen in water, a concentrated jelly containing starch grains.
Foaming
The foaming process is the ability of proteins to form highly concentrated liquid-gas systems called foams. The stability of the foam, in which the protein is a foaming agent, depends not only on its nature and concentration, but also on temperature. Proteins are widely used as foaming agents in the confectionery industry (marshmallow, marshmallow, soufflé). Bread has a foam structure, and this affects its taste properties.
Combustion
Proteins burn with the formation of nitrogen, carbon dioxide and water, as well as some other substances. Burning is accompanied by the characteristic smell of burnt feathers.
color reactions.
- Xantoprotein - interaction of aromatic and heteroatomic cycles in a protein molecule with concentrated nitric acid occurs, accompanied by the appearance of a yellow color;
- Biuret - there is an interaction of weakly alkaline solutions of proteins with a solution of copper (II) sulfate with the formation of complex compounds between Cu 2+ ions and polypeptides. The reaction is accompanied by the appearance of a violet-blue color;
- when proteins are heated with alkali in the presence of lead salts, a black precipitate forms, which contains sulfur.
- How to take B vitamins
- Reactions of glucose by alcohol groups
- What is the difference between ethyl alcohol and methyl alcohol
- What is the need for blood
- C6h12o6 chemical properties
- Methods for obtaining metals
- How to remove itching, redness and swelling?
- Methods for examining the intestines without colonoscopy
- Drugs. Signs of use. Help for drug addicts. The main signs of drug addiction The three main signs of drug addiction
- The main symptoms of the disease
- How is the treatment of psychoses and neuroses of varying severity?
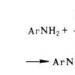
- Amines - concept, properties, application
- What can be transmitted through saliva
- Chemical properties of proteins
- Pills for fever
- How to protect the liver when using drugs?
- Vitamin A deficiency: reasons for what to do
- Which is better: Theraflu or Coldrex?
- Ulcerative colitis of the intestine - symptoms, treatment, causes
- Lichen sclerosus: causes, symptoms and treatment Lichen disease