What color does medical alcohol burn? What is the difference between ethyl alcohol and methyl alcohol. Symptoms of technical alcohol poisoning
Alcohol, which we used to drink at birthdays, weddings and for relaxation, basically contains ethyl alcohol, supplemented with dyes and other components. In a diluted amount, it is approved for consumption and is widely used in the food and medical industries. There is another option, it is called methyl alcohol. It is from methyl alcohol that surrogate alcohol is made, which leads to severe poisoning and death. So that the alcohol consumed does not provoke a fatal outcome, it is very important to know how to distinguish ethyl alcohol from methyl alcohol.
Characteristic features and differences of alcohols
Ethyl and methyl alcohol differ not only in their chemical formula, but also in a number of other characteristics. Alcohol made on the basis of ethyl alcohol does not have devastating consequences, if you consume it in reasonable quantities, you can even get some benefit, which cannot be said about the effect of methyl alcohol. It is used for surrogates, as it is much cheaper than the food option. Methyl alcohol is also called technical alcohol, it is often used to create organic dyes and glass.
Methyl alcohols can be poisonous, even if a small amount has entered the bloodstream. Negative changes in well-being are noted after the use of 30 grams of methanol.
Few people know how to distinguish methyl alcohol, in principle it is not strange, because it is difficult to recognize them by color and smell, they are identical, and the formula is not displayed on the bottle. You can tell the difference if you have a good sense of smell. Methyl alcohol has a less intense odor, and ethyl enhances any odors, respectively, opening the bottle and catching a strong aroma, we can assume that there is ethyl. Unfortunately, few people manage to distinguish the substance by smell, respectively, and people experience blindness, vomiting and asthma attacks by using methyl.
Among the differences, it can be noted that ethyl has a disinfecting effect, it is lighter than water and can easily ignite. Methyl liquids burn worse, have less pronounced disinfecting properties and are able to draw in water.
If you do not know how to check what alcohol your drink consists of, you can set it on fire, alcohol burns in different colors. A green flame will help to identify methyl, ethyl can only burn blue.
Signs of methyl poisoning

In the home, determining the quality of a drink may involve inspecting a bottle of alcohol. If the label is pasted crookedly, there are spelling errors on the label, or there is no information about the manufacturer and excise stamp, it is likely that this is a fake.
You can buy a drink only at verified points of sale, which have the appropriate licenses and certificates of compliance with international standards.
To detect methyl, you can also pay attention to well-being. Of course, this is the most extreme way, but it has been tested more than once. Having soaked a finger in a liquid, they lick it and wait half an hour. If a person develops negative symptoms, then it is likely that you have tried a substance burning with a green flame. Among the bright signs of methyl poisoning are:
- dizziness;
- vomiting;
- nausea;
- fainting state;
- impaired concentration;
- pupil dilation;
- redness of the skin, an allergic reaction is possible;
- bouts of coughing and choking;
- tremor in the limbs;
- pallor of the skin.
From ethyl, used in such a small amount, there are no such serious consequences. After a person has checked the liquid in this way, he needs to be artificially induced - vomiting, and an enema. It is advisable to drink activated charcoal with the calculation of 1 tablet per 1 kg of weight. If the symptoms do not stop and the condition worsens, you need to seek medical help.
Simple ways to distinguish between ethyl and methyl

The easiest way to determine methyl alcohol in alcohol is to immerse a peeled potato in liquid. If the potato turns pink in half an hour, it is likely that it has been in contact with methanol. In case the color change does not occur or the potato turns blue, the alcohol contains a certain percentage of ethyl.
You can distinguish methyl alcohol from ethyl alcohol using potassium permanganate. Alcohol at home is poured into a container that can be heated. Add a little potassium permanganate and heat to 18 degrees. Next, it is necessary to detect the time of change in the color of the liquid. It can be colored from purple to yellow-pink. The test is considered passed, and alcohol with ethanol is in the container if discoloration is detected no earlier than 10 minutes.
Among other, no less simple ways, how to distinguish methanol from ethanol, the following experiments can be noted:
- The difference between ethyl alcohol and alcohol methanol can be seen if the liquid is heated. Technical alcohols have a lower boiling point. That is, in the methyl version, boiling at 64 degrees can be detected, and ethyl alcohol needs a high temperature, about 78 degrees.
- Specifying how ethyl alcohol differs from methyl alcohol, one cannot forget about the school experiment with chemistry. A heated copper wire is immersed in a cold liquid. If during the reaction of copper, the smell of rotten apples is felt or the smell of vinegar is determined, then the wire has come into contact with ethyl alcohol. The difference is felt almost immediately when the same copper wire is lowered into a container of alcohol based on methyl. There will be a pungent smell of formalin.
- Cooks know how to determine ethyl alcohol, they add a little baking soda to the liquid, if an insoluble thick yellow precipitate appears at the bottom, then the soda was dipped into ethyl. By lowering the soda into the methylene liquid, the precipitate will be white or transparent.
- You can distinguish methyl using potassium permanganate, if you add a small amount of it during the boiling of the liquid, the difference will be that ethyl does not form bubbles, but methyl forms.
Which of the above methods for determining alcohols, you would not choose, remember that they do not give a 100% guarantee. To accurately distinguish what substance your drink is full of, laboratory staff can, by carrying out chemical reactions.
If you want to maintain your health for a long time, it is better to completely abandon the use of strong drinks, and not look for differences between "bad" and "even worse." There is not much difference between the intoxicant, ethyl kills a person slowly, destroying internal organs, and methyl is distinguished by the fact that it kills instantly. And in the first, and in the second alcoholic drink - death awaits, remember this, and lead a healthy lifestyle.
Alcohol is the main ingredient in almost all alcoholic beverages. But in order to end up with high-quality, tasty, and most importantly safe products, you need to choose the right alcohol.
Alcohol can be of two types: ethyl and methyl. Which of them can be drunk or eaten, and what color they burn, we will tell below.
Ethyl or ethanol

Ethyl alcohol is of two types: food and medical. It is suitable for consumption, however, before that it must be diluted with water, otherwise, due to the high concentration of alcohol, serious burns of the digestive tract and oral cavity can be obtained.
Without special manipulations, "by eye" it is impossible to distinguish methyl alcohol from ethyl alcohol. Both liquids are transparent, with a characteristic odor, the same density. True, methyl has a neutral aroma, which is rather weakly expressed and is felt only when “sniffing”, while ethanol vapor is sharper and blows into the nose.
Such a product is obtained by distillation of the wort, which has previously passed the fermentation stage. You can also get it yourself at home, however, for this you need to use a special distillate - moonshine.
Reference. Homemade moonshine, made from high-quality raw materials and in accordance with technological rules, is in no way inferior in quality to industrial alcohol.
But it should be understood that with prolonged use of this ethyl product in large quantities, alcohol dependence may appear. Therefore, all drinks prepared on its basis must be consumed in small quantities.
Methyl or methanol

Methyl or technical alcohol, which is usually simply called methanol by the people- This is an industrial product, it cannot be used for food in any form. This liquid is obtained from legnin, formic alcohol and wood.
Use such a product for the manufacture of solvents and formaldehyde. When working with such a substance, you should definitely follow safety precautions, since methanol has a strong and pungent aroma, and can also cause severe burns to the epidermis.
Reference. Technical alcohol is absorbed into the body much more slowly than ethanol, it destroys the nervous system, causes severe poisoning, and also impairs vision.
That is why, when making your own alcohol-based alcoholic beverages, it is very important to use the right product.
When buying alcoholic beverages in stores with a proven reputation, the risk of poisoning with a “scorched” product is significantly reduced. Most often, drinks with methyl alcohol are sold in stalls, stalls, from hands, at unauthorized points of sale and drinking, as well as near railway stations.
How to define a species?

However, in order to independently determine which type of alcohol is represented, it is not at all necessary to carry its samples to the laboratory. There are several simple ways to determine the type of alcohol:
- All that is needed is to set fire to the liquid. You can pour a small amount into any container, or you can simply moisten the edge of a cotton swab and bring it to a fire source. This method does not require the use of any special tools, and the diagnostic result is instant. Ethyl alcohol always burns only with a blue fire, but the flame of methanol is green. But this rule only works for methyl alcohol without unnecessary impurities.
- There is also the so-called potato test. A small root crop is thoroughly cleaned and washed, then cut into two parts and both are placed in a jar. Pour potatoes with alcohol and leave for 2-5 hours. If after this period the water does not turn pink, then ethyl alcohol, and in the future it can be used.
- You can add a small amount of baking soda to a vessel with alcohol. If there is ethanol in the container, then the soda will turn yellow and settle at the bottom of the container. If the alcohol is methyl, then it will completely dissolve.
- Alcohol is slightly heated and a couple of granules of ordinary potassium permanganate are added to it. If the liquid is of high quality, then no changes will occur. If methanol was heated, then when potassium permanganate enters it, it will begin to hiss strongly and bubble.
- If you have a good industrial thermometer at hand, then a small amount of liquid can simply be heated in a metal bowl. At the moment when it starts to boil, its temperature should be measured. Ethanol boils at 80 degrees, and methyl alcohol begins to boil already at 60 degrees.
- Lang's test or discoloration of the liquid. To do this, in a small container, the test liquid is heated to 18 degrees. Manganese infusion is immediately poured here and everything is removed from the fire. Now you need to measure the time it takes to color the liquid from dark crimson to light pink. The longer this process takes place, the better the quality of the test liquid. The best option is 10 - 20 minutes. For such a sample, 50 g of alcohol, 2 g of water and 0.2 mg of potassium permanganate will be required.
- The simplest, but most reliable is the so-called formaldehyde study. All that is needed is to heat a copper wire over high heat and lower it into a container of liquid. After 15 seconds, the copper is removed and sniffed. The stronger the aroma of formaldehyde, the more low-quality alcohol, which means that you should never drink it.
Attention! When buying alcohol in random places, or, even more so, when you receive a homemade product as a gift, smell it before drinking. If the smell seems too harsh, somehow unusual, do not be too lazy to conduct a home examination in order to prevent severe poisoning.
Symptoms of poisoning and first aid

If methanol was nevertheless consumed, then this will be evidenced by:
- flickering before the eyes and deterioration of visibility;
- severe headache and dizziness;
- severe cutting cramps in the stomach;
- nausea and vomiting.
A person can also poorly navigate in space and lose consciousness. In such cases, immediately need to provide first aid:
- call an ambulance and explain that the victim has severe methanol poisoning.
- It is necessary to give the person a soda solution to drink. The volume of the drunk liquid should not be less than 1500 ml.
- You need to cleanse your intestines. To do this, first induce vomiting, and then put an enema.
For intoxication and neutralization of methanol, the victim is given to drink 50 ml of slightly diluted ethanol every three hours. But do not self-medicate at home. Only in a medical institution can assess the degree of poisoning and provide proper assistance.
Ethyl alcohol poisoning
You can also get poisoned with ethyl alcohol if you exceed the permissible dose of consumption. The amount of alcohol that enters the bloodstream is measured in ppm (‰) - a conventional unit denoting 1/10 of a percent of alcohol per 1 liter of blood.

First aid for ethyl alcohol poisoning (hangover)
If a drunkard feels sick, he should be given the opportunity to empty his stomach properly, and if he is able to drink water, it should be offered in sufficient quantities. The more alcohol the body expels from itself at this stage, the better. It is important to ensure that a person does not choke on drink or vomit. The next morning after the "victim" oversleep, he will need:
- One tablet of acetylsalicylic acid and analgin (or aspirin + citramon) to relieve headaches.
- Activated carbon, enterosgel or other sorbents.
- Plentiful drink - mineral water, compote, sweet drinks save well, because during the "libations" the body loses glucose.
- If there is an urge to vomit, you can cause it artificially by drinking a glass of clean water before that.
- Refreshing shower, airing the room, easy walk.
- Chicken broth, soup, some oatmeal, greens, or a raw egg will kick start your stomach and help you recover faster.
Heavy physical activity, exposure to contrasting temperatures, heavy food should be avoided and you should not “hangover”.

Video: how to check the quality of alcohol at home?
If there are doubts about the purchased alcoholic liquid, then it is better to do several studies at the same time to determine its quality. Watch a video that uses the Lang method - one of the easy ways to test the quality of alcohol at home:
There are several ways to distinguish ethanol from industrial alcohol at home. This does not require special equipment, fixtures, devices (with the exception of a thermometer).
Sample heating
After heating alcohol in a metal container on the fire of a home stove burner, it is necessary to measure the boiling point of the liquid. For ethyl alcohol, it is higher - (78С), for a technical analogue it is lower (64С).
Copper wire testing
Now it is not the test sample that will have to be heated, but the copper wire. To increase the contact area, it is better to roll it into a spiral.
Heating is necessary until white or black, so that copper oxide begins to stand out.
The red-hot wire is lowered into a liquid, the nature of which is determined by the smell: methanol has an unpleasant odor, ethyl alcohol smells like an apple (sweet, but distinguishable).
Chemical reaction method for manganese
For this test, you need a transparent container in which alcohol is placed. A few drops of potassium permanganate (or crystals) will cause a vinegar smell without bubbles in drinking alcohol. In methanol, the reaction will begin with the release of gases (bubbles).
Iodine reaction
You will need water heated to 50C, alkali (sodium hydroxide), a drop of iodine, alcohol. When mixing all the components in drinking alcohol, iodoform (yellow precipitate) should appear. In industrial alcohol, such a reaction is impossible - there will be no precipitate.
long-term way
In methyl alcohol, a raw peeled potato turns pink AFTER A FEW hours, but in drinking alcohol it does not change color.
All these methods can only be used for pure products. They are not suitable for their mixture. A person risks going blind from the use of methanol.
How to learn to speak correctly
In the modern world, people are increasingly paying attention to the culture of communication. IN...
Ways to fill female energy
It happens that fatigue gradually accumulates and breaks out through negative e...
Which is better for arthritis pain relief - ice or heat?
A short answer from a rheumatologist. Fire or ice - hot or cold. Q: What would be better for...
5 Effective Ways to Prevent Aggression
How to prevent aggression? In the wild nature, conflicts are resolved in one way - demon...
What is the difference between psoriasis and eczema
Itchy rash? How to tell if it's eczema or psoriasis. Similar symptoms require similar...
What to do - acne all over the body
Most of us have occasionally had pimples on our back or chest. Those nasty little ones...
Healing and healing properties of nettle
For most people, stinging nettle is still considered just a common weed. However, good...
If a child asks for a pet
Parents often have a huge number of reasons to refuse a child in ...
Finding low-quality alcohol in a container and drinking it is not so bad. Much worse to use instead of ethyl alcohol methyl. It is very difficult to distinguish them by eye, which leads to frequent poisoning. Methanol is the strongest poison that adversely affects the nervous and vascular systems, as well as vision. If a person survives, he often remains blind. Taking methyl alcohol causes lethargy, headache, general malaise, pain in the lower back and abdomen. Permissible loss of consciousness. Ingestion of 30 to 100 ml of methanol is fatal.
Instruction
1. Ethyl and methyl alcohol are ideally identical in taste, smell and color, so it will be quite difficult for an ordinary person to distinguish them. There are several ways to determine what is in front of you - ethanol and methanol. To establish the quality alcohol you can try lighting the liquid. Follow the color of the fire. If alcohol burns with a blue flame, then, most likely, you have ethanol in front of you. Methyl alcohol blazes green.
2. The folk method includes a sample using potatoes. Peel a raw potato and toss a small wedge into a bowl. After a few hours, it may change its color. If it turns pink, the alcohol being tested is methanol. In ethyl alcohol, potatoes actually do not change color.
3. One of the most reliable methods to check the chemical affiliation alcohol considered formaldehyde test. Take a copper wire and heat it up on a fire. Then dip it into the liquid. The methanol will give off a strong unpleasant smell of formaldehyde. Ethanol in such cases is virtually odorless or smells of a faint aroma of apples. Similar verification methods are also used in the final result. Moisten a cotton wool with alcohol, set it on fire and put it out coolly. You can also add potassium permanganate to the liquid and set it on fire. Determine the presence of the accessory from the above outgoing odors alcohol to the ethanol or methanol group.
Methyl alcohol is a compound belonging to the group of monohydric alcohols. Methanol is highly toxic, each 10 ml of this substance can cause severe damage to the central nervous system, blindness, and 30 ml - death. That is why there is a need for its identification. It is much easier to review methyl alcohol in a toxicological laboratory, but it is absolutely acceptable to make the simplest determination even at home.

Instruction
1. Methanol is colorless, smell and taste indistinguishable from ethyl alcohol. However, qualitatively these substances differ significantly. As a result, many poisonings occur. If the test solution contains only one alcohol, then it will not be difficult to determine which one. But if you have a mixture of alcohols or alcohol with impurities in front of you, then you can find out a good and quantitative table of contents only in laboratory conditions. To determine some alcohols (ethanol, glycerin), there is a good reaction - an iodoform test. It is carried out very first in order to verify or exclude the content of ethanol in methanol. As a result of the test, clear yellow crystals of triiodomethane (iodoform) precipitate. Methanol does not give this reaction. C? H? OH + J? + NaOH = CHJ?? + NaJ + HCOONa + H?O
2. Many good reactions to methyl alcohol are based on its transformation into methyl aldehyde (formaldehyde). Pour the solution into a test tube with a gas outlet tube, add potassium permanganate in the presence of sulfuric acid. As a result of distillation, formaldehyde is formed, which can be done with different reagents. Schiff's reagent gives a stubborn violet color, chromotropic acid - a violet color of the solution, potassium hexacyanoferrate - a blue-violet color, Felling's reagent - a black precipitate. These reactions are confirmatory for methanol.
3. At home, the survey can be carried out using copper wire. Heat it over the fire and dip it into the solution to be studied. If it contained methanol, then the smell of formalin will appear - cool and hefty unpleasant. There will be no such result with ethanol. The quantitative determination of the content of methanol is carried out in laboratory conditions by titrimetric methods and gas-liquid chromatography.
methanol and ethanol are clear liquids that are indistinguishable in taste. However, taking 10 ml of methyl alcohol, which is equal to 2 teaspoons in volume, can lead to severe poisoning, and 30 ml or more can be fatal. Consequently, it is not at all superfluous in everyday conditions to be able to distinguish one alcohol from another.

You will need
- - mug;
- - thermometer;
- - plate;
- - copper wire;
- - tincture of iodine;
- - potassium permanganate;
- - drinking soda;
- - potato.
Instruction
1. Take a metal mug and fill it one third with the liquid to be tested. Put on the stove and turn on the burner. Dip the thermometer in alcohol. Record the boiling point of the liquid, it is possible to assume the chemical composition of alcohol from it. Methyl alcohol boils at 64°C, ethyl alcohol at 78°C.
2. Heat a piece of copper wire over the flame of a lighter and dip it in alcohol. The copper oxide formed during heating will react with the test liquid. Among other reaction products, aldehyde will be present, which has a classic smell. If the test liquid is ethanol, you will smell vinegar or rotten apples. In the case of methanol, you will inhale formalin vapors, which annoyingly irritate the nasal mucosa.
3. Pour a small amount of alcohol into a transparent container, add a pinch of baking soda and stir thoroughly. Drop tincture of iodine into the resulting mixture. See if there is any sediment. Ethanol reacts with iodine to form iodoform, a yellow insoluble substance. methanol remains clear and does not precipitate.
4. Add a few crystals of potassium permanganate to alcohol and heat the pink solution. The release of gas bubbles indicates that you have methyl alcohol in front of you.
5. Try the popular method for determining the chemical composition of alcohol. Soak the peeled potatoes in the liquid for a few hours. A pink hue indicates that the alcohol is methyl, blue - ethyl.
Note!
The above methods will not be authentic if the methyl alcohol contains ethanol impurities and vice versa.
Helpful advice
Household methods for determining the chemical composition of alcohol are not unconditionally correct. The exact result is admissible only after the chromatographic review in the chemical laboratory.
A strong figure is, before anyone else, an individuality that manifests itself in any act, in work or communication. A strong person is not afraid of being different from others; on the contrary, he is drawn to self-expression.

Confidence, initiative, responsibility
Under all circumstances, a strong figure is confident in himself and his abilities. He believes that he will achieve his goals and get the desired result, while realistically assessing his abilities. A strong person continuously expands his probabilities, continuously improves himself. A weak figure, on the contrary, is not confident in himself and his abilities. Such a person is not really interested in anything. While doing something, he does not gravitate towards more and remains on the same tier, slowly degrading. A powerful person values himself and his abilities above everyone else, and a weak person values something that is outside of him. It can be money, location, connections, relatives. Strong people are not afraid of uncertainty in life, on the contrary, it stimulates them to knowledge and change. Preparedness for continuous internal and external changes is the source of their inner confidence. A strong person is convinced that everything in his life depends only on him. He does not try to win the approval of others. He takes full responsibility for his actions. Such a person does not believe in anyone, he considers himself the owner of his own destiny and does not demand anything from people.
Relationships with others, feelings
An integral quality of a strong person is the knowledge to establish favorable and great relationships with people. He accepts those around him as they are, without teaching or educating anyone, without bothering to subdue or apply anyone. It is very difficult for weak people to build relationships even with the closest ones. They do not know how to get from those around them what they need to satisfy their needs. A strong person understands that it is unrealistic to change the people around him without starting to change himself. This, in his judgment, leads to the achievement of the desired result. Weak people more often than anyone in communication use a limited number of behavioral patterns, therefore they are never satisfied with their relationships with others. Strong people openly show their feelings, both positive and negative. The weak ones try to hide behind a mask, only to be afraid of confessing their own weakness. Even to themselves, they do not admit what they are really experiencing. It is easy to communicate with powerful people, because they do not suffer from complexes and snags, they are cheerful and open. To the weak, on the contrary, a non-standard approach is invariably needed, and one has to adapt to them. A strong person is emotional to his own thoughts and experiences. He tries to allow all the internal strife and objections that arise in order to feel great. A weak figure lets everything take its course, thereby turning them into psychological complexes, neuroses, etc. A powerful person periodically feels the need for solitude, without feeling lonely at the same time. A weak person is sad with himself, he constantly finds it hard to go into a mob, trying to merge with it and forget about his inner emptiness. A powerful person is invariably optimistic, for him this is not connected with a physical disposition, affairs at work or someone else's judgment. Even getting into difficult situations, he does not lose his composure and optimism. Powerful people are not sensitive, do not harbor resentment in themselves, adequately respond to the situation.
Note!
If you accidentally ingest methyl alcohol, call your doctor immediately. The antidote in this case is 10% ethyl alcohol. Give it intravenously at the rate of 1-2 ml of 96% ethanol per kilogram of body weight. Suitable is the introduction of calcium salts and gastric lavage.
What are the differences between methyl and ethyl alcohols? This question can be addressed to the specialists of the chemical laboratory, who, in turn, will present a great amount of information that will not be understood by an ordinary person with basic knowledge. In fact, there is a huge difference between these two types of product, although they belong to the same class of organic chemistry - the group of alcohols. Let's leave the scientific point of view in place and move on to everyday issues ...
How to tell the difference between methyl and ethyl alcohol?
Methyl alcohol (CH3OH), you can often hear the name "methanol", in terms of visual characteristics it does not differ much from ethyl alcohol. It consists of a colorless liquid with a less pronounced but similar odour. Only on the first signs of methyl and ethyl alcohols can be confused. Methanol can also act as a fuel, but it has not gained much popularity in this area due to a significant drawback - the ignition temperature of methanol is too low, the content of strong and harmful poisons, alcohol is able to draw liquid. Methanol, unlike ethanol, is a life-threatening poison! When ingested, thirty grams of this alcohol can cause blindness, and fifty grams will lead to the death of the victim.Ethyl alcohol (C2H5OH). In simple terms, this is "ethanol" simple drinking alcohol. Taking in small doses, it serves as a dope for the human nervous system. Ethanol has the following properties:
- flammable.
- has a disinfecting effect.
- much lighter than water.
Ethyl alcohol has been widely used both as an alcoholic product and in industrial terms, cosmetics, cleaning products and many other areas surrounding a person.
Generally speaking, ethanol has received the widest use in production and everyday life, earning the status of the most valuable raw material. In addition, work is underway to replace gasoline and a number of other petroleum products used as fuel with ethyl alcohol.
Comparative characteristics of methyl and ethyl alcohols.
Unfortunately, there are no simple ways to distinguish methanol from ethanol. They are so similar in smell, taste and smell that only a highly skilled worker in the chemical industry can identify it by one species.
Along with this, there are a number of simple experiments that a person can conduct without leaving home. This may require the following: a burner, a container (metal), copper wire, a transparent test tube, or dishes, a thermometer (thermometer).
All of the above items can be easily found in stores.
Consider ways to recognize these two alcohols:
The first way: It is necessary to install a metal container with the tested alcohol on the burner, then measure the temperature at which this liquid begins to boil. Methyl alcohol will begin to boil at a temperature equal to sixty-four degrees Celsius. Ethyl alcohol, in turn, will begin to boil at seventy-eight degrees Celsius.The second method: It is necessary to heat the copper wire to white and place it in a bowl with the alcohol to be tested. After removing it from the container with alcohol, it is necessary to determine the smell of the gas that evaporates from the wire (it is necessary to carry out translational movements with the palms to the nose area). When you smell the aroma of rotten apples, you can confidently say that we have ethanol. If, on the contrary, the smell is annoyingly unpleasant and sharp - methanol.
It is worth noting that these methods can be carried out in relation to alcohols with a high concentration.
Based on the experiments, we can safely say that you should not take alcohol of unknown origin!
A few more ways to determine the difference between alcohols.
- The origin of the origin. It is necessary to purchase goods in stores that do not put you at risk of purchasing counterfeit products. It is better to buy products in specialized alcohol stores, and not at points of sale that cause you doubt.- Ignition method. With this method, everything is pretty simple. It is necessary to set fire to the test liquid and observe the color of the flame during combustion. You will see a blue flame - in front of you is ethyl alcohol, methyl alcohol burns with a green flame.
- How to test potatoes. A small piece of potato is placed in a bath with alcohol for several hours. If the potato acquires a pinkish hue, we can understand that we have methyl alcohol in front of us. If the potato remains without a change in color characteristics, we can say with accuracy that we have ethyl alcohol in front of us.
Symptoms of methanol poisoning.
The following symptoms can serve as methanol poisoning: nausea and vomiting, malaise, difficulty breathing, feeling lethargic and powerless, severe headache.
These signs are similar to those of ethanol poisoning and are often confused. But in cases of methanol poisoning, the outcome is most serious. Methyl instantly begins to destroy the vascular, visual and nervous systems of the human body.
In cases where, after drinking alcohol, a person becomes ill, you need to urgently call an ambulance.
- How to take B vitamins
- Reactions of glucose by alcohol groups
- What is the difference between ethyl alcohol and methyl alcohol
- What is the need for blood
- C6h12o6 chemical properties
- Methods for obtaining metals
- How to remove itching, redness and swelling?
- Methods for examining the intestines without colonoscopy
- Drugs. Signs of use. Help for drug addicts. The main signs of drug addiction The three main signs of drug addiction
- The main symptoms of the disease
- How is the treatment of psychoses and neuroses of varying severity?
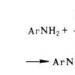
- Amines - concept, properties, application
- What can be transmitted through saliva
- Chemical properties of proteins
- Pills for fever
- How to protect the liver when using drugs?
- Vitamin A deficiency: reasons for what to do
- Which is better: Theraflu or Coldrex?
- Ulcerative colitis of the intestine - symptoms, treatment, causes
- Lichen sclerosus: causes, symptoms and treatment Lichen disease