C6h12o6 chemical properties. Carbohydrates. Hyperglycemia and hypoglycemia
1. General information
a) D-glucose - a -D-glucose - b -D-glucose
b) L-glucose
3. Being in nature
4. Receipt
5. Application
6. Physical properties
7. Chemical properties
8. Ribose and deoxyribose
9. Some interesting facts
10. Literature
Glucose formula C 6 H 12 O 6.
Glucose is a monosaccharide, one of the eight isomeric aldohexoses. Molar mass 180 g/mol. D-form glucose (dextose, grape sugar) is the most abundant carbohydrate. D-glucose (usually referred to simply as glucose) occurs in its free form and as oligosaccharides (cane sugar, milk sugar), polysaccharides (starch, glycogen, cellulose, dextran), glycosides, and other derivatives. In free form, D-glucose is found in fruits, flowers and other plant organs, as well as in animal tissues (blood, brain, etc.). D-glucose is the most important source of energy in animals and microorganisms. Like other monosaccharides, D-glucose comes in several forms. Crystalline D-glucose was obtained in 2 forms: a-D-glucose and b-D-glucose.
a-D-glucose
t pl 146 ° С D = + 112.2 ° (in water), crystallizes from water in the form of a monohydrate with t pl 83 ° С.
b-D-glucose
Obtained by crystallization of D-glucose from pyridine and some other solutions. t pl 148-150 ° C, D = + 18.9 ° (in water).
In an aqueous solution, an equilibrium is established between several interconvertible forms of D-glucose: a - and b -pyranose, a - and b -furanose, open aldehyde and hydrate forms. In an equilibrium system in water D = + 52.7°.
CHO S HCOH S HOCH S HCOH S HCOH S CH 2 OH
L-glucose
L-glucose is obtained synthetically by reduction of L-gluconic acid lactone. a -L-glucose - crystals t pl 142-143 ° C D \u003d - 95.5 ° (in water) and - 51.4 ° (equilibrium system in water). The chemical properties of L-glucose are the same as those of D-glucose.
Being in nature
In a special form, glucose is found in almost all organs of green plants. It is especially abundant in grape juice, which is why glucose is sometimes called grape sugar. Honey mainly consists of a mixture of glucose and fructose.
In the human body, glucose is found in the muscles, in the blood (0.1 - 0.12%) and serves as the main source of energy for the cells and tissues of the body. An increase in the concentration of glucose in the blood leads to an increase in the production of the pancreatic hormone - insulin, which reduces the content of this carbohydrate in the blood. The chemical energy of nutrients entering the body lies in the covalent bonds between atoms. In glucose, the amount of potential energy is 2800 kJ per 1 mole (that is, per 180 grams).
Receipt
The first synthesis of glucose from formaldehyde in the presence of calcium hydroxide was carried out by A. M. Butlerov in 1861: O / / Ca (OH) 2
6H-C * * ® C 6 H 12 O 6
\\ H Glucose can be obtained by hydrolysis of natural substances in which it is included. In production, it is obtained by hydrolysis of potato and corn starch with acids.
H 2 SO 4, t (C 6 H 10 O 5) n + n H 2 O * * ® n C6H12O6
The complete syntheses of glucose carried out starting from crolein dibrom, as well as from glyceraldehyde and dihydroxyacetone, are of only theoretical interest.
In nature, glucose, along with other carbohydrates, is formed as a result of the photosynthesis reaction: chlorophyll 6CO 2 + 6H 2 O * * * * ® C 6 H 12 O 6 + 6O 2 - Q
During this reaction, the energy of the Sun is accumulated.
Application
Glucose is a valuable nutritious product. In the body, it undergoes complex biochemical transformations resulting in the formation of carbon dioxide and water, while energy is released according to the final equation: C 6 H 12 O 6 + 6O 2 * ® 6H 2 O + 6CO 2 + 2800 kJ This process proceeds in steps, and so energy is released slowly.
Glucose is also involved in the second stage of the energy metabolism of an animal cell (glucose breakdown). The overall equation looks like this: C 6 H 12 O 6 + 2H 3 PO 4 + 2ADP s ® 2C 3 H 6 O 3 + 2ATP + 2H 2 O Since glucose is easily absorbed by the body, it is used in medicine as a strengthening remedy for phenomena heart weakness, shock, it is a part of blood-substituting and anti-shock liquids. Glucose is widely used in confectionery (making marmalade, caramel, gingerbread, etc.), in the textile industry as a reducing agent, as an initial product in the production of ascorbic and glyconic acids, for the synthesis of a number of sugar derivatives, etc.
Of great importance are the processes of fermentation of glucose. So, for example, when pickling cabbage, cucumbers, milk, lactic acid fermentation of glucose occurs, as well as when ensiling feed. If the mass being ensiled is not sufficiently compacted, butyric fermentation occurs under the influence of the infiltrated air and the feed becomes unsuitable for use.
In practice, alcoholic fermentation of glucose is also used, for example, in the production of beer.
Physical properties
Glucose is a colorless crystalline substance with a sweet taste, highly soluble in water. From an aqueous solution, it is released in the form of crystalline C 6 H 12 O 6 · H 2 O. Compared to beet sugar, it is less sweet.
Chemical properties
Glucose has chemical properties characteristic of alcohols and aldehydes. In addition, it also has some specific properties:
|
Properties due to the presence in the molecule |
Specific Properties |
|
|
hydroxyl groups |
aldehyde group |
|
|
1. Reacts with carboxylic acids to form esters (five hydroxyl groups of glucose react with acids) |
1. Reacts with silver oxide (I) in an ammonia solution (“silver mirror” reaction): CH 2 OH (CHOH) 4 -COH + Ag 2 O® CH 2 OH (CHOH) 4 -CO 2 H + 2AgЇ |
Glucose is able to undergo fermentation: a) alcoholic fermentation C 6 H 12 O 6® 2CH 3 -CH 2 OH + CO 2 b) lactic acid fermentation C 6 H 12 O 6® 2CH 3 -CHOH-COOH lactic acid |
|
2. How does a polyhydric alcohol react with copper (II) hydroxide to form copper (II) alcoholate |
2. Oxidized by copper (II) hydroxide (with a red precipitate) 3. Under the action of reducing agents, it turns into a six-hydric alcohol |
c) butyric fermentation C 6 H 12 O 6® C 3 H 7 COOH + 2H 2 + 2CO 2 butyric acid |
D-glucose gives general reactions to aldoses, it is a reducing sugar, forms a number of derivatives due to the aldehyde group (phenylhydrazone, n- bromophenylhydrazone, etc.). Glucose ozazone is identical to mannose ozonose, which is an epimer of glucose, and fructose ozazone. When glucose is reduced, the six-hydric alcohol sorbitol is formed; during the oxidation of the aldehyde group of glucose - monobasic D-gluconic acid, with further oxidation - dibasic D-sugar acid. When only the secondary alcohol group of glucose is oxidized (provided that the aldehyde group is protected), D-glucuronic acid is formed. The formation of D-glucuronic acid from D-glucose can occur under the action of glucose oxidase or dehydrogenase enzymes. During the pyrolysis of D-glucose, glycosans are formed: a-glycosan and levoglucosan (b-glucosan).
For the quantitative determination of glucose, calorimetric, iodometric and other methods are used.
Ribose and deoxyribose
Of the pentoses, ribose and deoxyribose are of great interest, because they are part of nucleic acids. The structural formulas of open chain ribose and deoxyribose are as follows: S S S S \ OH OH OH OH H OH OH OH H H ribose deoxyribose
Some interesting facts
Some frogs have found uses for glucose in their bodies - a curious, though much less important one. In winter, you can sometimes find frogs frozen into ice blocks, but after thawing, amphibians come to life. How do they manage not to freeze to death? It turns out that with the onset of cold weather, the amount of glucose in the blood of a frog increases 60 times. This prevents the formation of ice crystals inside the body.
glycolysis
The heroes of Jules Verne's novel "Children of Captain Grant" were just about to dine on the meat of a wild llama (guanaco) they had shot, when it suddenly turned out that it was completely inedible.
“Perhaps it lay too long?” - puzzled asked one of them.
“No, it, unfortunately, ran for too long! - answered the scientist-geographer Paganel - Guanaco meat is tasty only when the animal is killed during the rest, but if it is hunted for a long time and the animal ran for a long time, then its meat is inedible. It is unlikely that Paganel would have been able to explain the reason for the phenomenon he described. But, using the data of modern science, it is not at all difficult to do this. You will have to start, however, somewhat from afar.
When a cell breathes oxygen, glucose “burns” in it, turning into water and carbon dioxide, and releases energy. But suppose an animal runs for a long time, or a person quickly performs some hard physical work, for example, chopping firewood. Oxygen does not have time to get into the muscle cells. However, the cells do not "suffocate" immediately. A curious process begins - glycolysis (which means “splitting of sugar”). When glucose breaks down, it is not water and carbon dioxide that is formed, but a more complex substance - lactic acid. Everyone who has tried sour milk or kefir is familiar with its taste.
Energy during glycolysis is released 13 times less than during respiration. The more lactic acid accumulated in the muscles, the stronger the person or animal feels their fatigue. Finally, all muscle glucose stores are depleted. Rest is needed. Therefore, having stopped chopping wood or running up a long staircase, a person usually “takes a breath”, making up for the lack of oxygen in the blood. It was lactic acid that made the meat of the animal shot by the heroes of Jules Verne tasteless.
Literature
Brief Chemical Encyclopedia
Textbook Chemistry Grade 10
Encyclopedia for children - Biology
An organic oxygen-containing compound with six carbon atoms is called glucose, grape sugar, dextrose, or hexose. It is a universal source of energy among all living organisms on the planet. The formula of the substance is C 6 H 12 O 6.
Structure
The substance glucose in chemistry is a monosaccharide, that is, the simplest carbohydrate, consisting of one molecule or one structural unit. Glucose structural unit is part of more complex carbohydrates - disaccharides and polysaccharides.
Substance includes functional groups :
- one carbonyl (-C=O);
- five hydroxyl (-OH).
The molecule can exist in the form of two cycles (α and β), differing in the spatial arrangement of one hydroxyl group, and in a linear form (D-glucose).
Glucose takes the cyclic form in aqueous solutions.

Rice. 1. Cyclic and linear glucose molecule.
The structural formula of glucose is O \u003d CH-CHOH-CHOH-CHOH-CHOH-CH 2 OH or CH 2 OH (CHOH) 4 -COH.
Receipt
A large amount of grape sugar is found in vegetation, in particular fruits and leaves. Therefore, the substance can be consumed directly from fruits and berries. Glucose is the end product of photosynthesis:
6CO 2 + 6H 2 O → C 6 H 12 O 6 + 6O 2 - Q.
In industry, the compound is isolated by hydrolysis of polysaccharides. The initial products are potato or grain starch, as well as cellulose. A hot solution of sulfuric or hydrochloric acid is added to the raw material diluted with water. The resulting mixture is heated until the complete decomposition of polysaccharides:
(C 6 H 10 O 5) n + nH 2 O (t °, H 2 SO 4) → nC 6 H 12 O 6.
The acid is neutralized with chalk or porcelain, after which the solution is filtered and evaporated. The resulting crystals are glucose.

Rice. 2. Scheme for obtaining glucose.
In laboratories, dextrose is isolated from formaldehyde in the presence of a catalyst - Ca (OH) 2:
6H-CH=O → C 6 H 12 O 6 .
In the digestive tract, dietary polysaccharides are quickly broken down into fructose and glucose, which are involved in cellular metabolism.
Physical properties
Hexose is a colorless, odorless crystalline substance with a sweet taste. However, sucrose (habitual sugar) is twice as sweet as glucose.
The substance is highly soluble not only in water, but also in other solvents - an ammonia solution of copper hydroxide (Schweitzer's reagent), sulfuric acid, zinc chloride.
Chemical properties
Glucose combines the properties of aldehydes (contains the -CHO group) and alcohols (including hydroxyl), being an aldehyde alcohol. Therefore, it can form alcohol and polymerize similarly to aldehydes. The main chemical properties of glucose are described in the table.
|
Reaction |
Description |
The equation |
|
Fermentation |
It breaks down under the action of enzymes secreted by bacteria, fungi, yeast. There are three types depending on the nature of the enzyme: alcoholic, butyric, lactic acid fermentation |
|
|
silver mirror |
Qualitative reaction with an ammonia solution of silver oxide (I) with the formation of gluconic acid |
C 6 H 12 O 6 + Ag 2 O + NH 4 OH → CH 2 OH (CHOH) 4 -COOH + 2Ag ↓ |
|
Oxidation with nitric acid |
Glucose is oxidized to sugar (glucaric) acid |
C 6 H 12 O 6 + HNO 3 → C 6 H 10 O 8 |
|
Oxidation with copper(II) hydroxide |
Blue copper(II) hydroxide is converted to red copper(I) oxide. Gluconic acid is formed |
CH 2 OH (CHOH) 4 -COH + 2Cu (OH) 2 → CH 2 OH (CHOH) 4 -COOH + Cu 2 O + 2H 2 O |
|
Recovery |
Reaction with hydrogen in the presence of a catalyst (nickel) at high temperature. Hexahydric alcohol (sorbitol) is formed |
CH 2 OH (CHOH) 4 -COH + H 2 → CH 2 OH (CHOH) 4 -CH 2 OH |

Rice. 3. Scheme of glucose fermentation.
Glucose is used in medicine, food, textile industry. The substance is present in all food products, used to produce beer, wine, lactic acid products.
What have we learned?
Glucose is a monosaccharide containing six carbon atoms. It is formed as a result of photosynthesis, hydrolysis of polysaccharides, from formaldehyde. The functional groups are -C=O and -OH. In reactions, it shows the properties of aldehydes and alcohols. Reacts with an ammonia solution of silver, copper (II) hydroxide, nitric acid, hydrogen, undergoes alcoholic, butyric, lactic acid fermentation. Thanks to fermentation, food products are obtained - kefir, cheese, alcohol.
Topic quiz
Report Evaluation
Average rating: 4.4. Total ratings received: 262.
The most important of the monosaccharides is glucose C 6 H 12 O 6, which is otherwise called grape sugar. It is a white crystalline substance, sweet in taste, highly soluble in water. Glucose is found in plant and living organisms, its content is especially high in grape juice (hence the name - grape sugar), in honey, as well as in ripe fruits and berries.
The structure of glucose is derived from the study of its chemical properties. So, glucose exhibits properties inherent in alcohols: it forms alcoholates (saccharates) with metal, an acetic acid ester containing five acid residues (according to the number of hydroxyl groups). Therefore, glucose is a polyhydric alcohol. With an ammonia solution of silver oxide, it gives a "silver mirror" reaction, indicating the presence of an aldehyde group at the end of the carbon chain. Therefore, glucose is an aldehyde alcohol, its molecule can have the structure
However, not all properties are consistent with its structure as an aldehyde alcohol. So, glucose does not give some reactions of aldehydes. One hydroxyl out of five is characterized by the greatest reactivity, and the replacement of hydrogen in it by a methyl radical leads to the disappearance of the aldehyde properties of the substance. All this led to the conclusion that, along with the aldehyde form, there are cyclic forms of glucose molecules (α-cyclic and β-cyclic), which differ in the position of hydroxyl groups relative to the plane of the ring. The cyclic structure of the glucose molecule is in the crystalline state, while in aqueous solutions it exists in various forms that mutually transform into each other:

As you can see, the aldehyde group is absent in the cyclic forms. The hydroxyl group at the first carbon atom is the most reactive. The cyclic form of carbohydrates explains many of their chemical properties.
On an industrial scale, glucose is produced by the hydrolysis of starch (in the presence of acids). Its production from wood (cellulose) has also been mastered.
Glucose is a valuable nutrient. When it is oxidized in the tissues, the energy necessary for the normal functioning of organisms is released. The oxidation reaction can be expressed by the overall equation:
C 6 H 12 O 6 + 6 O 2 → 6CO 2 + 6H 2 O
Glucose is used in medicine for the preparation of medicinal preparations, blood preservation, intravenous infusion, etc. It is widely used in the confectionery industry, in the production of mirrors and toys (silvering). It is used for dyeing and finishing fabrics and leathers.
Glucose in Greek means "sweet". In nature, in large quantities, it is found in the juices of berries and fruits, including grape juice, which is why it is popularly called "wine sugar".
Discovery history
Glucose was discovered at the beginning of the 19th century by the English physician, chemist and philosopher William Prout. This substance gained wide popularity after Henri Braccono extracted it from sawdust in 1819.
Physical properties
Glucose is a colorless crystalline powder with a sweet taste. It is highly soluble in water, concentrated sulfuric acid, and Schweitzer's reagent.
The structure of the molecule
Like all monosaccharides, glucose is a heterofunctional compound (the molecule contains several hydroxyl and one carboxyl group). In the case of glucose, the carboxyl group is an aldehyde.
The general formula for glucose is C6H12O6. The molecules of this substance have a cyclic structure and two spatial isomers of alpha and beta forms. In the solid state, the alpha form predominates almost 100%. In solution, the beta form is more stable (it occupies approximately 60%). Glucose is the end product of the hydrolysis of all poly- and disaccharides, that is, the production of glucose occurs in the vast majority of cases in this way.
Getting a substance
In nature, glucose is formed in plants as a result of photosynthesis. Consider industrial and laboratory methods for obtaining glucose. In the laboratory, this substance is the result of aldol condensation. In industry, the most common way is to obtain glucose from starch.
Starch is a polysaccharide, the monoparts of which are glucose molecules. That is, to obtain it, it is necessary to decompose the polysaccharide into monoparts. How is this process carried out?
Obtaining glucose from starch begins with the fact that the starch is placed in a container of water and mixed (starch milk). Bring another container of water to a boil. It is worth noting that boiling water should be twice as much as starched milk. In order for the reaction to produce glucose to go to completion, a catalyst is needed. In this case, it is salt or The calculated amount is added to a container of boiling water. Then the starch milk is slowly poured in. In this process, it is very important not to get a paste, if nevertheless it is formed, boiling should be continued until it disappears completely. On average, boiling takes an hour and a half. In order to be sure that the starch is completely hydrolyzed, it is necessary to carry out a qualitative reaction. Iodine is added to the selected sample. If the liquid becomes blue in color, then the hydrolysis is not completed, but if it becomes brown or red-brown, then there is no more starch in the solution. But this solution contains not only glucose, it was obtained with the help of a catalyst, which means that acid also has a place to be. How to remove acid? The answer is simple: by neutralizing with pure chalk and finely crushed porcelain.
Neutralization is checked Next, the resulting solution is filtered. The point is small: the resulting colorless liquid should be evaporated. The formed crystals are our end result. Now consider the production of glucose from starch (reaction).
The chemical essence of the process
This equation for obtaining glucose is presented before the intermediate product - maltose. Maltose is a disaccharide consisting of two glucose molecules. It is clearly seen that the methods for obtaining glucose from starch and from maltose are the same. That is, in continuation of the reaction, we can put the following equation.

In conclusion, it is worth summarizing the necessary conditions for the successful production of glucose from starch.
The necessary conditions
- catalyst (hydrochloric or sulfuric acid);
- temperature (at least 100 degrees);
- pressure (atmospheric is enough, but increasing pressure speeds up the process).

This method is the simplest, with a high yield of the final product and minimal energy costs. But he's not the only one. Glucose is also obtained from cellulose.
Preparation from cellulose
The essence of the process almost completely corresponds to the previous reaction.

The preparation of glucose (formula) from cellulose is given. In fact, this process is much more complicated and energy-intensive. So, the reaction product is waste from the wood processing industry, crushed to a fraction, the particle size of which is 1.1 - 1.6 mm. This product is treated first with acetic acid, then with hydrogen peroxide, then with sulfuric acid at a temperature of at least 110 degrees and a hydromodulus of 5. The duration of this process is 3-5 hours. Then, for two hours, hydrolysis takes place with sulfuric acid at room temperature and hydromodulus 4-5. This is followed by dilution with water and inversion for about an hour and a half.
Quantification methods
Having considered all the methods for obtaining glucose, methods for its quantitative determination should be studied. There are situations when only a solution containing glucose should participate in the technological process, that is, the process of evaporating the liquid until crystals are obtained is superfluous. Then the question arises, how to determine what concentration of a given substance in a solution. The resulting amount of glucose in solution is determined by spectrophotometric, polarimetric and chromatographic methods. There is also a more specific method of determination - enzymatic (using the enzyme glucosidase). In this case, the calculation is already the products of the action of this enzyme.
Application of glucose
In medicine, glucose is used for intoxication (it can be both food poisoning and infection activity). In this case, the glucose solution is administered intravenously using a dropper. This means that in pharmacy, glucose is a universal antioxidant. Also, this substance plays an important role in the detection and diagnosis of diabetes mellitus. Here glucose acts as a stress test.
In the food industry and cooking, glucose occupies a very important place. Separately, the role of glucose in winemaking, beer and moonshine production should be indicated. We are talking about such a method as obtaining ethanol. Let us consider this process in detail.
Getting alcohol
Alcohol production technology has two stages: fermentation and distillation. Fermentation, in turn, is carried out with the help of bacteria. In biotechnology, cultures of microorganisms have long been bred, which allow you to get the maximum yield of alcohol with the minimum amount of time spent. In everyday life, ordinary table yeast can be used as reaction assistants.
First of all, glucose is diluted in water. The microorganisms used are diluted in another container. Further, the resulting liquids are mixed, shaken and placed in a container with This tube is connected to another one (U-shaped). In the middle of the second tube is poured. The end of the tube is closed with a rubber stopper with a hollow glass rod having a drawn end.

This container is placed in a thermostat at a temperature of 25-27 degrees for four days. Turbidity will be observed in a tube with lime water, which indicates that carbon dioxide has reacted with it. As soon as carbon dioxide ceases to be released, fermentation can be considered finished. Next comes the distillation step. In the laboratory for the distillation of alcohol, reflux condensers are used - devices in which cold water passes along the outer wall, thereby cooling the formed gas and transferring it back to a liquid.

At this stage, the liquid that is in our container should be heated to 85-90 degrees. Thus, the alcohol will evaporate, but the water will not be brought to a boil.
The mechanism for obtaining alcohol
Consider the production of alcohol from glucose in the reaction equation: C6H12O6 \u003d 2C2H5OH + 2CO2.
So, it can be noted that the mechanism for producing ethanol from glucose is very simple. Moreover, it has been known to mankind for many centuries, and brought almost to perfection.
The value of glucose in human life
So, having a certain understanding of this substance, its physical and chemical properties, use in various industries, we can conclude what glucose is. Obtaining it from polysaccharides already gives an understanding that, being the main component of all sugars, glucose is an indispensable source of energy for humans. As a result of metabolism, adenosine triphosphoric acid is formed from this substance, which is converted into a unit of energy.

But not all glucose that enters the human body goes to replenish energy. In the waking state, a person converts only 50 percent of the received glucose into ATP. The rest is converted to glycogen and stored in the liver. Glycogen breaks down over time, thereby regulating blood sugar levels. Quantitatively, the content of this substance in the body is a direct indicator of its health. The hormonal functioning of all systems depends on the amount of sugar in the blood. Therefore, it is worth remembering that excessive use of this substance can lead to serious consequences.
Glucose at first glance is a simple and understandable substance. Even from the point of view of chemistry, its molecules have a fairly simple structure, and the chemical properties are clear and familiar in everyday life. But, despite this, glucose is of great importance both for the person himself and for all spheres of his life.
Carbohydrates are organic substances whose molecules consist of carbon, hydrogen and oxygen atoms. Moreover, hydrogen and oxygen in them are in the same proportions as in water molecules (1: 2)
The general formula of carbohydrates is C n (H 2 O) m, i.e. they seem to consist of carbon and water, hence the name of the class, which has historical roots. It appeared on the basis of the analysis of the first known hydrocarbons. Later it was found that there are carbohydrates in the molecules of which there is no ratio 1H: 2O, for example, deoxyribose - C 5 H 10 O 4 . Organic compounds are also known, the composition of which fits the given general formula, but which do not belong to the class of carbohydrates. These include, for example, formaldehyde CH 2 O and acetic acid CH 3 COOH.
However, the name "hydrocarbons" has taken root and is generally recognized for these substances.
Hydrocarbons according to their ability to hydrolyze can be divided into three main groups: mono-, di- and polysaccharides.
Monosaccharides- carbohydrates that are not hydrolyzed (not decomposed by water). In turn, depending on the number of carbon atoms. Monosaccharides are classified into trioses(whose molecules contain three carbon atoms), tetroses(four atoms), pentoses(five), hexoses(six), etc.
In nature, monosaccharides are predominantly provided pentoses And hexoses. Pentoses include, for example, ribose C 5 H 10 O 5 and deoxyribose(ribose, from which the oxygen atom was “taken away”) C 5 H 10 O 4. They are part of RNA and DNA and determine the first part of the names of nucleic acids.
Hexoses having the general molecular formula C 6 H 12 O 6 include, for example, glucose, fructose, galactose.
disaccharides- carbohydrates that are hydrolyzed to form two molecules of monosaccharides, such as hexoses. The general formula of the vast majority of disaccharides is not difficult to deduce: you need to “add” the two hexose formulas and “subtract” the water molecule from the resulting formula - C 12 H 22 O 10. Accordingly, the general hydrolysis equation can also be written:
C 12 H 22 O 10 + H 2 O → 2C 6 H 12 O 6
Disaccharides include:
1) C sucrose(ordinary food sugar), which, when hydrolyzed, forms one glucose molecule and a fructose molecule. It is found in large quantities in sugar beets, sugar cane (hence the names - beet and cane sugar), maple (Canadian pioneers extracted maple sugar), sugar palm, corn, etc.
2) Maltose(malt sugar), which is hydrolyzed to form two molecules of glucose. Maltose can be obtained by hydrolysis of starch under the action of enzymes contained in malt - germinated, dried and ground barley grains.
3)Lactose(milk sugar), which is hydrolyzed to form glucose and galactose molecules. It is found in the milk of mammals, has a low sweetness, and is used as a filler in pills and pharmaceutical tablets.
The sweet taste of different mono- and disaccharides is different. So, the sweetest monosaccharide - fructose - is 1.5 times sweeter than glucose, which is taken as a standard. . sucrose(disaccharide), in turn, is 2 times sweeter than glucose, and 4-5 times sweeter than lactose, which is almost tasteless.
Polysaccharides - starch, glycogen, dextrins, cellulose, etc. - carbohydrates that are hydrolyzed to form many monosaccharide molecules, most often glucose.
To derive the formula of polysaccharides, it is necessary to “subtract” a water molecule from a glucose molecule and write an expression with the index n: (C 6 H 10 O 5) n. After all, it is precisely due to the elimination of water molecules that di- and polysaccharides are formed in nature.
The role of carbohydrates in nature and their price in human life is extremely important. Formed in plant cells as a result of photosynthesis, they act as a source of energy for animal cells. First of all, this applies to glucose.
Many carbohydrates (starch, glycogen, sucrose) perform a storage function, the role of a reserve of nutrients.
DNA and RNA acids, which include some carbohydrates (pentose-ribose and deoxyribose), perform the functions of transmitting hereditary information.
Cellulose - the building material of plant cells - plays the role of a framework for the membranes of these cells. Another polysaccharide chitin- performs a similar role in the cells of some animals: the outer skeleton of arthropods (crustaceans), insects, and arachnids is formed.
Carbohydrates are the ultimate source of our nutrition, whether we consume starchy grains or feed them to animals that convert starch into fats and proteins. The most hygienic clothes are made from cellulose or products based on it: cotton and linen, viscose fiber, acetate silk. Wooden houses and furniture are built from the same pulp that makes up wood. At the heart of the production of film and photographic film is the same cellulose. Books, newspapers, letters, banknotes - all these are products of the pulp and paper industry. This means that carbohydrates provide us with the most necessary for life: food, clothing, shelter.
In addition, carbohydrates are involved in the construction of complex proteins, enzymes, hormones. Carbohydrates are also such vital substances as heparin (it plays an important role - it prevents blood clotting), agar-agar (it is obtained from seaweed and used in the microbiological and confectionery industries - remember the famous Bird's Milk cake).
It must be emphasized that the only type of energy on Earth (besides nuclear, of course) is the energy of the Sun, and the only way to accumulate it to ensure the vital activity of all living organisms is the process of photosynthesis, which takes place in cells and leads to the synthesis of carbohydrates from water and carbon dioxide. It is during this transformation that oxygen is formed, without which life on our planet would be impossible:
6CO 2 + 6H 2 O → C 6 H 12 O 6 + 6O 2

Physical properties and being in nature
Glucose And fructose- solid and colorless substances crystalline substances. Glucose, found in grape juice (hence the name “grape sugar”), together with fructose, which is found in some fruits and vegetables (hence the name “fruit sugar”), makes up a significant portion of honey. The blood of humans and animals constantly contains about 0.1% glucose (80-120 mg per 100 ml of blood). Its largest part (about 70%) undergoes slow oxidation in the tissues with the release of energy and the formation of end products - water and carbon dioxide (glycolysis process):
C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + 2920 kJ
The energy released during glycolysis largely provides the energy needs of living organisms.
An increase in blood glucose levels of 180 mg per 100 ml indicates a violation of carbohydrate metabolism and the development of a dangerous disease - diabetes mellitus.
The structure of the glucose molecule
The structure of the glucose molecule can be judged on the basis of experimental data. It reacts with carboxylic acids to form esters containing 1 to 5 acid residues. If a glucose solution is added to freshly obtained copper hydroxide (||), then the precipitate dissolves and a bright blue solution of the copper compound is obtained, i.e., a qualitative reaction to polyhydric alcohols occurs. Hence , glucose is a polyhydric alcohol. If the resulting solution is heated, then a precipitate again falls out, then already reddish in color, i.e. there will be a qualitative response to aldehydes. Similarly, if a glucose solution is heated with an ammonia solution of silver oxide, then a “silver mirror” reaction will occur. Therefore, glucose is both a polyhydric alcohol and an aldehyde - aldehyde alcohol. Let's try to derive the structural formula of glucose. There are six carbon atoms in the C 6 H 12 O 6 molecule. One atom is part of the aldehyde group:
The remaining five atoms bind to hydroxyl groups. And finally, given that carbon is tetravalent, let's arrange the hydrogen atoms:
or:
However, it has been established that in addition to linear (aldehyde) molecules in a glucose solution, there are molecules of a cyclic structure that make up crystalline glucose. The transformation of molecules of a linear form into a cyclic one can be explained if we recall that carbon atoms can freely rotate around σ-bonds located at an angle of 109 about 28 / while the aldehyde group (1st carbon atom) can approach the hydroxyl group of the fifth carbon atom. In the first, under the influence of the hydroxy group, the π-bond is broken: a hydrogen atom is attached to the oxygen atom, and the oxygen of the hydroxy group that has "lost" this atom closes the cycle.
As a result of this rearrangement of atoms, a cyclic molecule is formed. The cyclic formula shows not only the order of bonding of atoms, but also their spatial arrangement. As a result of the interaction of the first and fifth carbon atoms, a new hydroxyl group appears at the first atom, which can occupy two positions in space: above and below the cycle plane, and therefore two cyclic forms of glucose are possible:
1) α-form of glucose - hydroxyl groups at the first and second carbon atoms are located on one side of the ring of the molecule;
2) β-forms of glucose - hydroxyl groups are located on opposite sides of the ring of the molecule:
In an aqueous solution of glucose, three of its isomeric forms are in dynamic equilibrium: the cyclic α-form, the linear (aldehyde) form, and the cyclic β-form.
In the established dynamic equilibrium, the β-form predominates (about 63%), since it is energetically preferable - it has OH groups at the first and second carbon atoms on opposite sides of the cycle. In the α-form (about 37%), the OH groups of the same carbon atoms are located on one side of the plane, so it is energetically less stable than the β-form. The share of the linear form in equilibrium is very small (only about 0.0026%).
The dynamic balance can be shifted. For example, when an ammonia solution of silver oxide acts on glucose, the amount of its linear (aldehyde) form, which is very small in solution, is replenished all the time due to cyclic forms, and glucose is completely oxidized to gluconic acid.
An isomer of the aldehyde alcohol of glucose is the keto alcohol fructose.
Chemical properties of glucose
The chemical properties of glucose, like any organic substance, are determined by its structure. Glucose has a dual function, being both an aldehyde and a polyhydric alcohol; therefore, it is characterized by the properties of both polyhydric alcohols and aldehydes.
Reactions of glucose as a polyhydric alcohol
Glucose gives a qualitative reaction of polyhydric alcohols (remember glycerol) with freshly prepared copper hydroxide (ǀǀ), forming a bright blue solution of a copper compound (ǀǀ).
Glucose, like alcohols, can form esters.
Reactions of glucose as an aldehyde
1. Oxidation of the aldehyde group. Glucose, as an aldehyde, is able to oxidize to the corresponding (gluconic) acid and give qualitative reactions to aldehydes. The reaction of the "Silver Mirror" (when heated):
CH 2 -OH-(CHOH) 4 -COH + Ag 2 O → CH 2 OH-(CHOH) 4 -COOH + 2Ag↓
Reaction with freshly obtained Cu (OH) 2 when heated:
CH 2 -OH-(CHOH) 4 -COH + 2 Cu(OH) 2 → CH 2 -OH-(CHOH) 4 -COOH + Cu 2 O↓ + H 2 O
2. Recovery of the aldehyde group. Glucose can be reduced to the corresponding alcohol (sorbitol):
CH 2 -OH-(CHOH) 4 -COH + H 2 → CH 2 -OH-(CHOH) 4 -CH 2 -OH
Fermentation reactions
These reactions proceed under the action of special biological catalysts of protein nature - enzymes.
1.Alcoholic fermentation:
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2
It has long been used by man to produce ethyl alcohol and alcoholic beverages.
2. Lactic fermentation:
which forms the basis of the vital activity of lactic acid bacteria and occurs during the souring of milk, pickling cabbage and cucumbers, ensiling green fodder



- How to take B vitamins
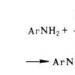
- Reactions of glucose by alcohol groups
- What is the difference between ethyl alcohol and methyl alcohol
- What is the need for blood
- C6h12o6 chemical properties
- Methods for obtaining metals
- How to remove itching, redness and swelling?
- Methods for examining the intestines without colonoscopy
- Drugs. Signs of use. Help for drug addicts. The main signs of drug addiction The three main signs of drug addiction
- The main symptoms of the disease
- How is the treatment of psychoses and neuroses of varying severity?
- Amines - concept, properties, application
- What can be transmitted through saliva
- Chemical properties of proteins
- Pills for fever
- How to protect the liver when using drugs?
- Vitamin A deficiency: reasons for what to do
- Which is better: Theraflu or Coldrex?
- Ulcerative colitis of the intestine - symptoms, treatment, causes
- Lichen sclerosus: causes, symptoms and treatment Lichen disease