Amines react with water. Amines - concept, properties, application. Obtaining amines in industrial conditions
N, e.g. CH 3 NH 2 -methylamine, CH 3 NHC 3 H 7 - methylpropylamine, (C 2 H 5) 3 N -. Names formed by adding the prefix "", "", etc. are also used. to the generic designation, for example, a compound of the type C 2 H 5 CH (NH 2) CH 2 CH 3 - 3-aminopentane. Many aromatic have trivial names, eg. C 6 H 5 NH 2 -, CH 3 C 6 H 4 NH 2 - and CH 3 OS 6 H 4 NH 2 - (respectively from "" and from ""). Higher aliphatic normal structure sometimes called. according to the names of fatty radicals to-t, from which they were synthesized, for example. , trilaurylamine.
In the IR spectra, the characteristic stretching vibrations of NH bonds in p-re are observed for primary alkylamines in the regions of 3380-3400 cm -1 and 3320-3340 cm -1; for primary aromatics. - two absorption bands in the region of 3500-3300 cm -1 (due to symmetric and asymmetric stretching vibrations of N-H bonds); for aliphatic and aromatic. secondary amiov-one band resp. in the region of 3360-3310 cm -1 and in the region of 3500-3300 cm -1 ; tertiaries do not absorb in this region. In the spectra of chem. the shift is 1-5 ppm. Aliphatic in the UV and visible regions do not absorb, aromatic. in the UV spectra they have two absorption bands due to -transitions.
When loading with carboxylic acids, theirs, anhydride chlorides or primary and secondary are acylated to form N-substituted amides, for example: RNH 2 + CH 3 COOH -> RNHCOCH 3 + H 2 O. react under mild conditions, even easier -, to-rymi spend in the presence. , binding formed in the district of HC1. When with dicarboxylic acids, their esters or are formed. Acylated ones have weak basic sv-you.
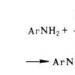
Under the action of HNO 2 aliphatic. the primary ones are converted into with the release of N 2 and H 2 O, the secondary ones are converted into N-nitrosamines R 2 NNO. Tertiary at normal t-re with HNO 2 do not react. R-tion with HNO 2 is used for aliphatic. . When interacting primary aromatics. with HNO 2 in an acidic environment are formed: ArNH 2 + HNO 2 + HC1 -> ArCl - + 2H 2 O. Under the same conditions, secondary aromatic. turn into N-nitrosamines, tertiary - into para-nitroso derivatives. Primary alicyclic. with HNO 2 they form, which is often accompanied by a narrowing or expansion of the cycle (see).
Aliphatic primary and secondary interactions. with C1 2 or Br 2, forming N-halogenated. Primary with COC1 2 form RNCO or disubstituted (RNH) 2 CO, secondary - tetrasubstituted R 2 NCONR 2 . Primary easy interaction. with , giving azomethines (), e.g.:
When interacting with primary and secondary, hydroxyethyl derivatives are formed, for example: C 6 H 5 NH 2 + C1CH 2 CH 2 OH -> C 6 H 5 NHCH 2 CH 2 OH + HCl. More often for the synthesis of the same Comm. apply, lesko reacting with in the presence. small quantities of H 2 O: 
When used instead of NH 3 primary or secondary, secondary and (or) tertiary are obtained. This method () is common for the production of N-alkyl- and N, N-dialkylanilines. A similar method for obtaining interaction has been developed. with NH3. They react very easily with NH 3, forming (see).
5. R-tion of amides aliphatic. and aromatic. carboxylic to-t with alkaline solutions C1 2, Br 2 or I 2 with the formation of primary. In this case, the carbon chain is shortened by one ().
6. R-tion with the participation of alkyl- and aryl halides. K with alkyl halides with last. (see) get pure primary aliphatic: 
Aryl halides react with NH 3 and with difficulty, so in the industry they use Comm., in which it is activated by strong electron-withdrawing substituents, most often nitro or sulfo groups. In this way, dec.
Organic bases - this name is often used in chemistry for compounds that are derivatives of ammonia. Hydrogen atoms in its molecule are replaced by hydrocarbon radicals. We are talking about amines - compounds that repeat the chemical properties of ammonia. In our article, we will get acquainted with the general formula of amines and their properties.
The structure of the molecule
Depending on how many hydrogen atoms are replaced by hydrocarbon radicals, primary, secondary and tertiary amines are distinguished. For example, methylamine is a primary amine in which the hydrogen moiety has been replaced with a -CH 3 group. The structural formula of amines is R-NH 2 and can be used to determine the composition of organic matter. An example of a secondary amine can be dimethylamine, having the following form: NH 2 -NH-NH 2 . In the molecules of tertiary compounds, all three hydrogen atoms of ammonia are replaced by hydrocarbon radicals, for example, trimethylamine has the formula (NH 2) 3 N. The structure of amines affects their physical and chemical properties.
Physical characteristic
The aggregate state of amines depends on the molar mass of the radicals. The smaller it is, the lower the specific gravity of the substance. The lower substances of the amine class are represented by gases (for example, methylamine). They have a pronounced smell of ammonia. Medium amines are low-smelling liquids, and compounds with a large mass of hydrocarbon radical are odorless solids. The solubility of amines also depends on the mass of the radical: the larger it is, the worse the substance dissolves in water. Thus, the structure of amines determines their physical state and characteristics.
Chemical properties
The characteristics of substances depend mainly on the transformations of the amino group, in which the leading role is assigned to its unshared electron pair. Since organic substances of the amine class are derivatives of ammonia, they are capable of reactions characteristic of NH 3. For example, compounds are soluble in water. The products of such a reaction will be substances that exhibit the properties of hydroxides. For example, methylamine, whose atomic composition obeys the general formula of saturated amines R-NH 2, forms a compound with water - methylammonium hydroxide:
CH 3 - NH 2 + H 2 O \u003d OH
Organic bases interact with inorganic acids, while salt is found in the products. So, methylamine with hydrochloric acid gives methylammonium chloride:
CH 3 -NH 2 + HCl -> Cl
The reactions of amines, the general formula of which is R-NH 2, with organic acids proceed with the replacement of the hydrogen atom of the amino group by a complex anion of the acid residue. They are called alkylation reactions. As in the reaction with nitrite acid, acyl derivatives can only form primary and secondary amines. Trimethylamine and other tertiary amines are not capable of such interactions. We also add that alkylation in analytical chemistry is used to separate mixtures of amines; it also serves as a qualitative reaction for primary and secondary amines. Among cyclic amines, aniline occupies an important place. It is extracted from nitrobenzene by reduction of the latter with hydrogen in the presence of a catalyst. Aniline is a raw material for the production of plastics, dyes, explosives and medicines.

Features of tertiary amines
Tertiary ammonia derivatives differ in their chemical properties from one- or two-substituted compounds. For example, they can interact with halogen derivatives of saturated hydrocarbons. As a result, tetraalkylammonium salts are formed. Silver oxide reacts with tertiary amines, while the amines are converted into tetraalkylammonium hydroxides, which are strong bases. Aprotic acids, such as boron trifluoride, are capable of forming complex compounds with trimethylamine.
Qualitative test for primary amines
Nitrous acid can serve as a reagent with which one or disubstituted amines can be detected. Since it does not exist in a free state, to obtain it in solution, a reaction is first carried out between dilute hydrochloric acid and sodium nitrite. The dissolved primary amine is then added. The composition of its molecule can be expressed using the general formula of amines: R-NH 2. This process is accompanied by the appearance of molecules of unsaturated hydrocarbons, which can be determined by reaction with bromine water or a solution of potassium permanganate. The isonitrile reaction can also be considered qualitative. In it, primary amines interact with chloroform in a medium with an excess concentration of hydroxo group anions. As a result, isonitriles are formed, which have an unpleasant specific odor.

Features of the reaction of secondary amines with nitrite acid
The technology for obtaining the HNO 2 reagent is described by us above. Then, an organic ammonia derivative containing two hydrocarbon radicals is added to the solution containing the reagent, for example, diethylamine, the molecule of which corresponds to the general formula of secondary amines NH 2 -R-NH 2 . In the reaction products we find a nitro compound: N-nitrosodiethylamine. If it is acted upon with hydrochloric acid, then the compound decomposes into the chloride salt of the starting amine and nitrosyl chloride. We also add that tertiary amines are not capable of reacting with nitrous acid. This is explained by the following fact: nitrite acid is a weak acid, and its salts, when interacting with amines containing three hydrocarbon radicals, are completely hydrolyzed in aqueous solutions.

How to get
Amines, whose general formula is R-NH 2 , can be obtained by reducing compounds containing nitrogen. For example, this can be the reduction of nitroalkanes in the presence of a catalyst - metallic nickel - when heated to +50 ⁰C and at a pressure of up to 100 atm. Nitroethane, nitropropane or nitromethane is converted into amines by this process. Substances of this class can also be obtained by hydrogen reduction of compounds of the nitrile group. This reaction takes place in organic solvents and requires the presence of a nickel catalyst. If metallic sodium is used as a reducing agent, in this case the process is carried out in an alcoholic solution. Let us give two more methods as examples: amination of haloalkanes and alcohols.

In the first case, a mixture of amines is formed. Amination of alcohols is carried out in the following way: a mixture of methanol or ethanol vapors with ammonia is passed over calcium oxide, which acts as a catalyst. The resulting primary, secondary and tertiary amines can usually be separated by distillation.
In our article, we studied the structure and properties of nitrogen-containing organic compounds - amines.
Since amines, being derivatives of ammonia, have a structure similar to it (i.e., they have an unshared pair of electrons in the nitrogen atom), they exhibit properties similar to it. Those. amines, like ammonia, are bases, since the nitrogen atom can provide an electron pair to form a bond with electron-deficient particles according to the donor-acceptor mechanism (corresponding to the definition of Lewis basicity).
I. Properties of amines as bases (proton acceptors)
1. Aqueous solutions of aliphatic amines show an alkaline reaction, because when they interact with water, alkylammonium hydroxides are formed, similar to ammonium hydroxide:
CH 3 NH 2 + H 2 O CH 3 NH 3 + + OH -


Aniline practically does not react with water.
Aqueous solutions are alkaline in nature:
The bond of a proton with an amine, as with ammonia, is formed according to the donor-acceptor mechanism due to the lone electron pair of the nitrogen atom.
Aliphatic amines are stronger bases than ammonia, because alkyl radicals increase the electron density on the nitrogen atom due to + I-effect. For this reason, the electron pair of the nitrogen atom is held less firmly and interacts more easily with the proton.
2. Interacting with acids, amines form salts:
C 6 H 5 NH 2 + HCl → (C 6 H 5 NH 3) Cl
phenylammonium chloride

2CH 3 NH 2 + H 2 SO 4 → (CH 3 NH 3) 2 SO 4
methyl ammonium sulfate
Amine salts are solids that are highly soluble in water and poorly soluble in non-polar liquids. When reacting with alkalis, free amines are released:
Aromatic amines are weaker bases than ammonia, since the lone electron pair of the nitrogen atom shifts towards the benzene ring, conjugating with the π-electrons of the aromatic nucleus, which reduces the electron density on the nitrogen atom (-M effect). On the contrary, the alkyl group is a good electron density donor (+I-effect).
 or
or 

A decrease in the electron density on the nitrogen atom leads to a decrease in the ability to split off protons from weak acids. Therefore, aniline interacts only with strong acids (HCl, H 2 SO 4), and its aqueous solution does not color litmus blue.
The nitrogen atom in amine molecules has an unshared pair of electrons, which can participate in the formation of a bond by the donor-acceptor mechanism.
aniline ammonia primary amine secondary amine tertiary amine
the electron density on the nitrogen atom increases.
Due to the presence of a lone pair of electrons in the molecules, amines, like ammonia, exhibit basic properties.
aniline ammonia primary amine secondary amine
the basic properties are enhanced, due to the influence of the type and number of radicals.
C6H5NH2< NH 3 < RNH 2 < R 2 NH < R 3 N (в газовой фазе)
II. Amine oxidation
Amines, especially aromatic ones, are easily oxidized in air. Unlike ammonia, they are capable of being ignited by an open flame. Aromatic amines spontaneously oxidize in air. Thus, aniline quickly turns brown in air due to oxidation.
4CH 3 NH 2 + 9O 2 → 4CO 2 + 10H 2 O + 2N 2
4C 6 H 5 NH 2 + 31O 2 → 24CO 2 + 14H 2 O + 2N 2
III. Interaction with nitrous acid
Nitrous acid HNO 2 is an unstable compound. Therefore, it is used only at the moment of selection. HNO 2 is formed, like all weak acids, by the action of a strong acid on its salt (nitrite):
KNO 2 + HCl → HNO 2 + KCl
or NO 2 - + H + → HNO 2
The structure of the reaction products with nitrous acid depends on the nature of the amine. Therefore, this reaction is used to distinguish between primary, secondary and tertiary amines.
Primary aliphatic amines with HNO 2 form alcohols:
R-NH 2 + HNO 2 → R-OH + N 2 + H 2 O
- Of great importance is the reaction of diazotization of primary aromatic amines under the action of nitrous acid obtained by the reaction of sodium nitrite with hydrochloric acid. And then phenol is formed:


Secondary amines (aliphatic and aromatic) under the action of HNO 2 are converted into N-nitroso derivatives (substances with a characteristic odor):
R 2 NH + H-O-N=O → R 2 N-N=O + H 2 O
alkylnitrosamine
· The reaction with tertiary amines leads to the formation of unstable salts and is of no practical importance.
IV. Special properties:
1. Formation of complex compounds with transition metals:
2. Addition of alkyl halides Amines add haloalkanes to form a salt:

By treating the resulting salt with alkali, you can get a free amine:

V. Aromatic electrophilic substitution in aromatic amines (reaction of aniline with bromine water or nitric acid):
In aromatic amines, the amino group facilitates substitution in the ortho and para positions of the benzene ring. Therefore, aniline halogenation occurs rapidly even in the absence of catalysts, and three hydrogen atoms of the benzene ring are replaced at once, and a white precipitate of 2,4,6-tribromaniline precipitates:

This reaction with bromine water is used as a qualitative reaction for aniline.
In these reactions (bromination and nitration) predominantly formed ortho- And pair-derivatives.
4. Methods for obtaining amines.
1. Hoffmann reaction. One of the first methods for obtaining primary amines is the alkylation of ammonia with alkyl halides:
This is not the best method, since the result is a mixture of amines of all degrees of substitution:
etc. Not only alkyl halides, but also alcohols can act as alkylating agents. To do this, a mixture of ammonia and alcohol is passed over aluminum oxide at high temperature.
2. Zinin's reaction- a convenient way to obtain aromatic amines in the reduction of aromatic nitro compounds. The following are used as reducing agents: H 2 (on a catalyst). Sometimes hydrogen is generated directly at the moment of the reaction, for which metals (zinc, iron) are treated with dilute acid.

2HCl + Fe (shavings) → FeCl 2 + 2H
C 6 H 5 NO 2 + 6 [H] C 6 H 5 NH 2 + 2H 2 O.
In industry, this reaction proceeds by heating nitrobenzene with water vapor in the presence of iron. In the laboratory, hydrogen "at the moment of isolation" is formed by the reaction of zinc with alkali or iron with hydrochloric acid. In the latter case, anilinium chloride is formed.
3. Recovery of nitriles. Use LiAlH 4:
4. Enzymatic decarboxylation of amino acids:

5. The use of amines.
Amines are used in the pharmaceutical industry and organic synthesis (CH 3 NH 2, (CH 3) 2 NH, (C 2 H 5) 2 NH, etc.); in the production of nylon (NH 2 - (CH 2) 6 -NH 2 - hexamethylenediamine); as a raw material for the production of dyes and plastics (aniline), as well as pesticides.
List of sources used:
- O.S. Gabrielyan and others. Chemistry. Grade 10. Profile level: textbook for educational institutions; Bustard, Moscow, 2005;
- "Tutor in Chemistry" edited by A. S. Egorov; "Phoenix", Rostov-on-Don, 2006;
- G. E. Rudzitis, F. G. Feldman. Chemistry 10 cells. M., Education, 2001;
- https://www.calc.ru/Aminy-Svoystva-Aminov.html
- http://www.yaklass.ru/materiali?mode=lsntheme&themeid=144
- http://www.chemel.ru/2008-05-24-19-21-00/2008-06-01-16-50-05/193-2008-06-30-20-47-29.html
- http://cnit.ssau.ru/organics/chem5/n232.htm
Amines- these are organic compounds in which the hydrogen atom (maybe more than one) is replaced by a hydrocarbon radical. All amines are divided into:
- primary amines;
- secondary amines;
- tertiary amines.
There are also analogues of ammonium salts - quaternary salts of the type [ R 4 N] + Cl - .
Depending on the type of radical amines can be:
- aliphatic amines;
- aromatic (mixed) amines.
Aliphatic limiting amines.
General formula C n H 2 n +3 N.
The structure of amines.
The nitrogen atom is in sp 3 hybridization. On the 4th non-hybrid orbital is a lone pair of electrons, which determines the main properties of amines:

Electron donor substituents increase the electron density on the nitrogen atom and enhance the basic properties of amines, for this reason, secondary amines are stronger bases than primary ones, because 2 radicals at the nitrogen atom create a greater electron density than 1.
In tertiary atoms, the spatial factor plays an important role: since 3 radicals obscure the lone pair of nitrogen, which is difficult to "approach" to other reagents, the basicity of such amines is less than primary or secondary ones.
Isomerism of amines.
Amines are characterized by isomerism of the carbon skeleton, isomerism of the position of the amino group:

What is the name of the amines?
The name usually lists hydrocarbon radicals (in alphabetical order) and adds the ending -amine:

Physical properties of amines.
The first 3 amines are gases, the middle members of the aliphatic series are liquids, and the higher ones are solids. The boiling point of amines is higher than that of the corresponding hydrocarbons, because in the liquid phase, hydrogen bonds are formed in the molecule.
Amines are highly soluble in water; as the hydrocarbon radical grows, the solubility decreases.
Getting amines.
1. Alkylation of ammonia (the main method), which occurs when an alkyl halide is heated with ammonia:
If the alkyl halide is in excess, then the primary amine can enter into an alkylation reaction, turning into a secondary or tertiary amine:
2. Recovery of nitro compounds:
Ammonium sulfide is used Zinin reaction), zinc or iron in an acidic environment, aluminum in an alkaline environment, or hydrogen in the gas phase.
3. Recovery of nitriles. use LiAlH 4:
4. Enzymatic decarboxylation of amino acids:

Chemical properties of amines.
All amines- strong bases, and aliphatic ones are stronger than ammonia.

Aqueous solutions are alkaline in nature.
Amines
Classification and nomenclature
Amines are organic derivatives of ammonia, in the molecule of which one, two or three hydrogen atoms are replaced by radicals. On this basis, one distinguishes primary (RNH 2) secondary (R 2 NH) and tertiary (R 3 N) amines.
Depending on the nature of the radical, amines can be limiting or aromatic, as well as limiting aromatic (methylamine, aniline and methylaniline, respectively). A branched radical can also be attached to the nitrogen atom (for example, tert butylamine), and polycondensed, which is demonstrated by the example of adamantylamine (aminoadamantane), which has a biological effect and is used in medicine


According to the principles of rational nomenclature, the name of this class of substances consists of the name of the radicals at the nitrogen atom, called amine. In the name of primary amines according to the international nomenclature, the amine nitrogen atom is given the name ami-But, used with its location before the name of the hydrocarbon chain. However, many amines retained their trivial names, for example, aniline".
In addition to the amino group, other substituents may also be present in the molecules of organic substances, as is the case, for example, in the case of sulfanilic acid. The amine nitrogen atom can also be included in the saturated cycle. Saturated heterocyclic amines include a three-membered strain-constructed ethyleneimine, with strong mutagenic activity. The ethyleneimine cycle is part of the molecules of some drugs. The tetrahydropyrrole and piperidine rings present in the molecules of a number of alkaloids (including nicotine and anabasine, see Section 20.4) are built without strain. With their participation, as well as with the help of the morpholine ring, the molecules of many drugs are built.




Heterocyclic aromatic amines are, for example, pyrrole and pyridine. Finally, the amino group can also be linked to a heterocycle, which is illustrated by the example of adenine (6-aminopurine), an indispensable fragment of nucleic acids.


Ammonia derivatives also include organic substances that can be built from ammonium salts or its hydroxide by replacing all four hydrogen atoms with various hydrocarbon radicals, as can be seen in the example of tetramethylammonium hydroxide:

Another example of tetrasubstituted ammonium derivatives - quaternary ammonium bases or their salts - is neuron, a toxic substance formed during the decay of animal tissues.
The quaternary nitrogen atom can be part of heterocycles, for example, the corresponding salt from the pyridine series - N-alkylpyridinium salt. These quaternary salts include some alkaloids. In addition, the quaternary nitrogen atom is part of many medicinal substances and some biomolecules.
The above examples demonstrate the diversity of amino compounds and their great biomedical significance. To this it must be added that the amino group is part of such classes of biomolecules as amino acids and proteins, nucleic acids, and is present in a number of natural derivatives of carbohydrates called amino sugars. The amino group is the most important functional group of alkaloids and numerous drugs for various purposes. Some examples of such substances will be given below.
24.3.2. Amines as organic bases

 The presence of a free electron pair of nitrogen gives amines the properties of bases. Therefore, a characteristic feature of amines is the reaction with acids with the formation of the corresponding ammonium salts, as can be seen from the reaction for the primary limiting amine:
The presence of a free electron pair of nitrogen gives amines the properties of bases. Therefore, a characteristic feature of amines is the reaction with acids with the formation of the corresponding ammonium salts, as can be seen from the reaction for the primary limiting amine:
Similarly, aniline salt is formed from aniline, pyridinium salt from pyridine, etc. Like ammonia, amines in aqueous solutions create an alkaline environment, according to the equation:
Quantitatively, the basicity of nitrogen-containing bases in an aqueous medium is reflected by the value of the equilibrium constant (TO b ) (more often use the value RK b ) ilip / C a (BH +), characterizing the acidity of the conjugate acid of a given base.

The strongest bases will be compounds containing a nitrogen atom, in which the lone pair of nitrogen is in the lone 5p 3 hybrid orbital (aliphatic amines, ammonia, amino acids), and the weakest ones will be those in which this pair participates in p, p-conjugation ( amides, pyrrole, pyridine).
Electron donor substituents, which include alkyl groups, should increase the basicity of amines, since they increase the electron density at the nitrogen atom. Yes, methylamine (pK b = 3.27) is a stronger base than ammonia (pK b = 4.75), and dimethylamine (pK b = 3.02) is a stronger base than methylamine. However, on going to trimethylamine, contrary to expectation, the basicity drops somewhat. (pK b = 4.10). The reason for this is that as the number of substituents on the nitrogen atom increases, the approach of the proton becomes more and more difficult. Thus, here we are not talking about the electronic, but the spatial effect of the substituents. This effect of substituents is called steric factor.
Aromatic amines are weaker bases than saturated ones due to the electron-withdrawing effect of the aromatic ring. Therefore, the basicity of pyridine is also low. The accumulation of phenyl substituents noticeably suppresses the activity of the electron pair of the nitrogen atom. So, pK, diphenylamine is 13.12, and triphenylamine does not show the properties of the base at all.
The extremely low basicity of pyrrole is due to the fact that in its molecule the electron pair of the nitrogen atom is involved in the formation of a bl-electron aromatic bond. Its binding with a proton requires a significant additional expenditure of energy. As a result of the formation of pyrrole salts, the aromatic bond and, consequently, the stability of the molecule disappear. This explains the fact that pyrrole in an acidic environment is quickly resinified.
It is interesting to note that the strong electron-withdrawing effect exerted by the pyrrole ring on the nitrogen atom leads to a weakening of the N-H bond, due to which pyrrole is able to exhibit the properties of a weak acid. (pK A = 17,5).
![]()
Under the action of such an active metal as potassium, its potassium salt, pyrrole-potassium, can be prepared.
The acidic properties of the N–H bond of the pyrrole ring explain, in particular, the ability of porphin and its natural derivatives to form salts with metal cations. Two pyrrole rings of the porphyrin molecule are coordinated with the cation due to the electron pairs of their nitrogen atoms, and the other two - replacing hydrogen atoms, like the molecule of pyrrole itself during the formation of pyrrole-potassium. These salts are chlorophyll and hemoglobin.

- How to take B vitamins
- Reactions of glucose by alcohol groups
- What is the difference between ethyl alcohol and methyl alcohol
- What is the need for blood
- C6h12o6 chemical properties
- Methods for obtaining metals
- How to remove itching, redness and swelling?
- Methods for examining the intestines without colonoscopy
- Drugs. Signs of use. Help for drug addicts. The main signs of drug addiction The three main signs of drug addiction
- The main symptoms of the disease
- How is the treatment of psychoses and neuroses of varying severity?
- Amines - concept, properties, application
- What can be transmitted through saliva
- Chemical properties of proteins
- Pills for fever
- How to protect the liver when using drugs?
- Vitamin A deficiency: reasons for what to do
- Which is better: Theraflu or Coldrex?
- Ulcerative colitis of the intestine - symptoms, treatment, causes
- Lichen sclerosus: causes, symptoms and treatment Lichen disease