Oxidation of palmitic acid. Energy balance of oxidation of saturated fatty acids with an even number of carbon atoms. How to increase the amount of fatty acids in your diet
100 RUR bonus for first order
Select the type of work Diploma work Course work Abstract Master's thesis Practice report Article Report Review Test work Monograph Problem solving Business plan Answers to questions Creative work Essay Drawing Essays Translation Presentations Typing Other Increasing the uniqueness of the text Master's thesis Laboratory work On-line help
Find out the price
Fatty acids are both saturated and unsaturated higher carboxylic acids, the hydrocarbon chain of which contains more than 12 carbon atoms. In the body, fatty acid oxidation is an extremely important process, and it can be directed to the α, β and ω carbon atoms of carboxylic acid molecules. Among these processes, β-oxidation occurs most frequently. It has been established that the oxidation of fatty acids occurs in the liver, kidneys, skeletal and cardiac muscles, and in adipose tissue. In brain tissue, the rate of fatty acid oxidation is very low; The main source of energy in brain tissue is glucose.
In 1904, F. Knoop put forward the hypothesis of β-oxidation of fatty acids based on experiments in feeding dogs various fatty acids in which one hydrogen atom in the terminal methyl group (ω-carbon atom) was replaced by a radical (C6H5– ).
Fatty acids, which are part of the natural fats of animals and plants, have an even number of carbon atoms. Any such acid from which a pair of carbon atoms is eliminated eventually passes through the butyric acid stage. After another β-oxidation, butyric acid becomes acetoacetic acid. The latter is then hydrolyzed to two molecules of acetic acid. The theory of β-oxidation of fatty acids, proposed by F. Knoop, largely served as the basis for modern ideas about the mechanism of fatty acid oxidation.
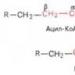
β-Oxidation of fatty acids. Carboxylic acids formed during the hydrolysis of fats undergo β-oxidation in mitochondria, where they enter in the form of the corresponding acyl coenzymes A. β-Oxidation is 4 successive ORPs.
I reaction. Dehydrogenation
// dehydrogenase /
C15H31 – CH2 – CH2 – C + FAD C = C + FAD(2H)
SCoA H COSCoA
Steryl coenzyme A is a trans isomer of steryl coenzyme A
II reaction Hydration
/ hydratase //
C = C + H2O C15H31 – CH – CH2 – C
H COSCoA OH SCoA
Trans isomer of steryl coenzyme A L-isomer of β-hydroxycarboxylic acid
III reaction Dehydrogenation
// dehydrogenase //
C15H31 – CH – CH2 – C + NAD+ C15H31 – C – CH2 – C + NADH + H+
OH SCoA O SCoA
β-oxoacid
IV reaction. Split
// thiolase // //
C15H31 – C – CH2 – C + HSCoA C15H31 – C CH3 – C
About SCoA SCoA SCoA
Palmitocoenzyme A Acetylcoenzyme A
On what's new in the Krebs cycle for
β-oxidation of final
oxidation
to CO2 and H2O
The four reactions of the β-oxidation process considered represent a cycle during which the carbon chain is shortened by two carbon atoms. Palmitocoenzyme A undergoes β-oxidation again, repeating this cycle. During the β-oxidation of one molecule of stearic acid, 40 ATP molecules are formed, including the Krebs cycle, which oxidizes the resulting acetyl coenzyme A - 146 ATP molecules. This indicates the importance of the processes of fatty acid oxidation from the point of view of the body’s energy.
α-Oxidation of fatty acids. In plants, under the action of enzymes, fatty acids are oxidized at the α-carbon atom - α-oxidation. This is a cycle consisting of two reactions.
I reaction consists of the oxidation of a fatty acid with hydrogen peroxide with the participation of the corresponding peroxidase into the corresponding aldehyde and CO2.
Peroxidase //
R – CH2 – COOH + 2 H2O2 R – C + CO2
As a result of this reaction, the carbon chain is shortened by one carbon atom.
II reaction consists of hydration and oxidation of the resulting aldehyde into the corresponding carboxylic acid under the action of aldehyde dehydrogenase with the oxidized form of NAD+:
// aldehyde- //
R – C + H2O + NAD+ dehydrogenase R – C + NAD(H) + H+
The α-oxidation cycle is characteristic only of plants.
ω-Oxidation of fatty acids. In the liver of animals and some microorganisms there is an enzyme system that provides ω-oxidation, i.e. oxidation at the terminal CH3 group. First, under the action of monooxygenase, hydroxylation occurs to form an ω-hydroxy acid:
ω monooxygenase
CH3 – R – COOH + “O” HOCH2 – R – COOH
HOCH2 – R – COOH + H2O + 2NAD+ dehydrogenase HOOC– R – COOH + 2 NAD (H) + 2H+
ω-dicarboxylic acid
The resulting ω-dicarboxylic acid is shortened at either end by a β-oxidation reaction.
If a carboxylic acid has branches, then its biological oxidation stops when it reaches the point of chain branching.
The process of fatty acid oxidation consists of the following main stages.
Activation of fatty acids. Free fatty acid, regardless of the length of the hydrocarbon chain, is metabolically inert and cannot undergo any biochemical transformations, including oxidation, until it is activated. Activation of the fatty acid occurs on the outer surface of the mitochondrial membrane with the participation of ATP, coenzyme A (HS-KoA) and Mg 2+ ions. The reaction is catalyzed by the enzyme acyl-CoA synthetase:
As a result of the reaction, acyl-CoA is formed, which is the active form of the fatty acid.
First stage of dehydrogenation. Acyl-CoA in mitochondria first undergoes enzymatic dehydrogenation, and acyl-CoA loses 2 hydrogen atoms in the α- and β-positions, turning into the CoA ester of an unsaturated acid.

Hydration stage. Unsaturated acyl-CoA (enoyl-CoA), with the participation of the enzyme enoyl-CoA hydratase, attaches a water molecule. As a result, β-hydroxyacyl-CoA (or 3-hydroxyacyl-CoA) is formed:

Second stage of dehydrogenation. The resulting β-hydroxyacyl-CoA (3-hydroxyacyl-CoA) is then dehydrogenated. This reaction is catalyzed by NAD+-dependent dehydrogenases:

Thiolase reaction. is the cleavage of 3-oxoacyl-CoA by the thiol group of the second CoA molecule. As a result, an acyl-CoA shortened by two carbon atoms and a two-carbon fragment in the form of acetyl-CoA are formed. This reaction is catalyzed by acetyl-CoA acyltransferase (β-ketothiolase):

The resulting acetyl-CoA undergoes oxidation in the tricarboxylic acid cycle, and acyl-CoA, shortened by two carbon atoms, again repeatedly goes through the entire β-oxidation path until the formation of butyryl-CoA (4-carbon compound), which in turn is oxidized up to 2 molecules of acetyl-CoA.
Energy balance. Each cycle of β-oxidation produces one molecule of FADH 2 and one molecule of NADH. The latter, in the process of oxidation in the respiratory chain and associated phosphorylation, give: FADH 2 - 2 ATP molecules and NADH - 3 ATP molecules, i.e. in total, 5 ATP molecules are formed in one cycle. The oxidation of palmitic acid produces 5 x 7 = 35 ATP molecules. In the process of β-oxidation of palmitic acid, 8 molecules of acetyl-CoA are formed, each of which, “burning” in the tricarboxylic acid cycle, gives 12 molecules of ATP, and 8 molecules of acetyl-CoA will give 12 x 8 = 96 molecules of ATP.
Thus, in total, with complete β-oxidation of palmitic acid, 35 + 96 = 131 ATP molecules are formed. Taking into account one ATP molecule spent at the very beginning on the formation of the active form of palmitic acid (palmitoyl-CoA), the total energy yield for the complete oxidation of one palmitic acid molecule under animal conditions will be 131 – 1 = 130 ATP molecules.
Hydrolysis triglycerides carried out by pancreatic lipase. Its optimum pH = 8, it hydrolyzes TG predominantly in positions 1 and 3, with the formation of 2 free fatty acids and 2-monoacylglycerol (2-MG). 2-MG is a good emulsifier. 28% of 2-MG is converted to 1-MG by isomerase. Most of the 1-MG is hydrolyzed by pancreatic lipase to glycerol and fatty acid. In the pancreas, pancreatic lipase is synthesized together with the protein colipase. Colipase is formed in an inactive form and is activated in the intestine by trypsin through partial proteolysis. Colipase, with its hydrophobic domain, binds to the surface of the lipid droplet, and its hydrophilic domain helps bring the active center of pancreatic lipase as close as possible to TG, which accelerates their hydrolysis.
|
Brown adipose tissue |
|
|
Quantity |
Little in an adult, high in a newborn |
|
Localization |
In its pure form: near the kidneys and thyroid gland. Mixed adipose tissue: between the shoulder blades, on the chest and shoulders. |
|
Blood supply |
Very good |
|
The structure of adipocytes |
There are many small droplets of fat in the cytoplasm, the nucleus and organelles are located in the center of the cell, there are many mitochondria and cytochromes. |
|
thermogenesis |
|
Oxidation occurs in the mitochondrial matrix. First the fatty acid is activated: 1 .In the cytoplasm of each acid is activated using CoA-8H and ATP energy. 2. The active fatty acid, acyl-CoA, is transported from the cytosol into the mitochondrial matrix (MC). CoA-8H remains in the cytosol, and the fatty acid residue - acyl - combines with carnitine (from the Latin - carnitine - meat - carnitine is isolated from muscle tissue) to form acyl-carnitine, which enters the intermembrane space of the mitochondria. From the intermembrane space of mitochondria, the acyl-carnitine complex is transferred into the mitochondrial matrix. In this case, carnitine remains in the intermembrane space. In the matrix, the acyl combines with CoA-8H. 3. Oxidation. An active fatty acid is formed in the MC matrix, which subsequently undergoes oxidation reactions to final products. In beta oxidation, the CH2- group in the beta position of the fatty acid is oxidized to the C- group. In this case, dehydrogenation occurs in two stages: with the participation of acyl dehydrogenase (flavin enzyme, hydrogen is transferred to ubiquinone) and beta-hydroxyacyl dehydrogenase (hydrogen acceptor NAD+). Then beta-ketoacyl-CoA, under the action of the enzyme thiolase, breaks down into acetyl CoA and acyl-CoA, shortened by 2 carbon atoms compared to the original. This acyl-CoA again undergoes beta-oxidation. Repeated repetition of this process leads to complete breakdown of the fatty acid to acyl-CoA. Oxidation of fatty acids. Includes 2 stages: 1. sequential cleavage of a two-carbon fragment in the form of acetyl-CoA from the C-terminus of the acid; 2. oxidation of acetyl-CoA in the Krebs cycle to CO2 and H2O. Energy value of fatty acid oxidation. Stearic acid (C 18) undergoes 8 oxidation cycles with the formation of 9 acetyl-CoA. In each oxidation cycle, 8 * 5 ATP = 40 ATP are formed, acetyl-CoA produces 9 * 12 ATP = 108 ATP. Total: 148 ATP, but 1 ATP is spent on activation of fatty acid in the cytosol, so the total is 147 ATP
β - oxidation of higher fatty acids (HFAs). Energy efficiency of the process (for saturated and unsaturated fatty acids). The influence of tissue oxidation of IVFA on the utilization of glucose by tissues.
β-oxidation - a specific pathway of catabolism of fatty acids with unbranched medium and short hydrocarbon chains. β-oxidation occurs in the mitochondrial matrix, during which 2 C atoms are sequentially separated from the C end of the FA in the form of Acetyl-CoA. β-oxidation of FA occurs only under aerobic conditions and is a source of large amounts of energy. β-oxidation of FA occurs actively in red skeletal muscles, cardiac muscle, kidneys and liver. FAs do not serve as a source of energy for nervous tissues, since FAs do not pass through the blood-brain barrier, like other hydrophobic substances. β-oxidation of FAs increases in the post-absorptive period, during fasting and physical work. At the same time, the concentration of FAs in the blood increases as a result of the mobilization of FAs from adipose tissue.
LCD activation
Activation of FA occurs as a result of the formation of a high-energy bond between FA and HSCoA with the formation of Acyl-CoA. The reaction is catalyzed by the enzyme Acyl-CoA synthetase:
RCOOH + HSKoA + ATP → RCO~SCoA + AMP+ PPn
Pyrophosphate is hydrolyzed by the enzyme pyrophosphatase: H 4 P 2 O 7 + H 2 O → 2H 3 PO 4
Acyl-CoA synthetases are found both in the cytosol (on the outer membrane of mitochondria) and in the mitochondrial matrix. These enzymes differ in their specificity for FAs with different hydrocarbon chain lengths.
Transport LCD. Transport of FAs into the mitochondrial matrix depends on the length of the carbon chain.
FAs with short and medium chain lengths (from 4 to 12 C atoms) can penetrate into the mitochondrial matrix by diffusion. Activation of these FAs occurs by acyl-CoA synthetases in the mitochondrial matrix. Long-chain FAs are first activated in the cytosol (by acyl-CoA synthetases on the outer mitochondrial membrane), and then transferred to the mitochondrial matrix by a special transport system using carnitine. Carnitine comes from food or is synthesized from lysine and methionine with the participation of vitamin C.
In the outer membrane of mitochondria, the enzyme carnitine acyltransferase I (carnitine palmitoyltransferase I) catalyzes the transfer of acyl from CoA to carnitine to form acylcarnitine;
Acylcarnitine passes through the intermembrane space to the outer side of the inner membrane and is transported by carnitine acylcarnitine translocase to the inner surface of the inner mitochondrial membrane;
The enzyme carnitine acyltransferase II catalyzes the transfer of acyl from carnitine to intramitochondrial HSCoA to form Acyl-CoA;
Free carnitine is returned to the cytosolic side of the inner mitochondrial membrane by the same translocase.
Reactions β-oxidation of FA
1. β-oxidation begins with the dehydrogenation of acyl-CoA by FAD-dependent acyl-CoA dehydrogenase, forming a double bond (trans) between the α- and β-C atoms of Enoyl-CoA. Reduced FADN 2, oxidizing in CPE, ensures the synthesis of 2 ATP molecules;
2. Enoyl-CoA hydratase adds water to the double bond of Enoyl-CoA to form β-hydroxyacyl-CoA;
3. β-hydroxyacyl-CoA is oxidized by NAD-dependent dehydrogenase to β-ketoacyl-CoA. Reduced NADH 2, oxidizing into CPE, ensures the synthesis of 3 ATP molecules;
4. Thiolase with the participation of HCoA cleaves Acetyl-CoA from β-ketoacyl-CoA. As a result of 4 reactions, Acyl-CoA is formed, which is shorter than the previous Acyl-CoA by 2 carbons. The formed Acetyl-CoA, oxidized in the TCA cycle, ensures the synthesis of 12 ATP molecules in the CPE.
Acyl-CoA then again enters into β-oxidation reactions. The cycles continue until Acyl-CoA turns into Acetyl-CoA with 2 C atoms (if the FA had an even number of C atoms) or Butyryl-CoA with 3 C atoms (if the FA had an odd number of C atoms).
Energy balance of oxidation of saturated fatty acids with an even number of carbon atoms
When FA is activated, 2 macroergic bonds of ATP are expended.
During the oxidation of a saturated FA with an even number of C atoms, only FADH 2, NADH 2 and Acetyl-CoA are formed.
During 1 cycle of β-oxidation, 1 FADH 2 , 1 NADH 2 and 1 Acetyl-CoA are formed, which upon oxidation produce 2 + 3 + 12 = 17 ATP.
Number of cycles during β-oxidation of FA = number of C atoms in (FA/2)-1. During β-oxidation, palmitic acid undergoes (16/2)-1 = 7 cycles. In 7 cycles, 17*7=119 ATP is formed.
The last cycle of β-oxidation is accompanied by the formation of additional Acetyl-CoA, which upon oxidation produces 12 ATP.
Thus, the oxidation of palmitic acid produces: -2+119+12=129 ATP.
Summary equation for β-oxidation, palmitoyl-CoA:
C 15 H 31 CO-CoA + 7 FAD + 7 NAD + + 7 HSKoA → 8 CH 3 -CO-KoA + 7 FADH 2 + 7 NADH 2
Energy balance of oxidation of saturated fatty acids with an odd number of carbon atoms
β-oxidation of a saturated FA with an odd number of C atoms at the beginning proceeds in the same way as with an even number. 2 macroergic bonds of ATP are spent on activation.
FA with 17 C atoms undergoes β-oxidation 17/2-1 = 7 cycles. In 1 cycle, 2+3+12=17 ATP is formed from 1 FADN 2, 1 NADH 2 and 1 Acetyl-CoA. In 7 cycles, 17*7=119 ATP is formed.
The last cycle of β-oxidation is accompanied by the formation not of Acetyl-CoA, but of Propionyl-CoA with 3 C atoms.
Propionyl-CoA is carboxylated at the cost of 1 ATP by propionyl-CoA carboxylase to form D-methylmalonyl-CoA, which, after isomerization, is converted first to L-methylmalonyl-CoA and then to Succinyl-CoA. Succinyl-CoA is included in the TCA cycle and, upon oxidation, produces PCA and 6 ATP. PIKE can enter gluconeogenesis for glucose synthesis. Vitamin B12 deficiency leads to the accumulation of methylmalonyl in the blood and excretion in the urine. During the oxidation of FA, the following is formed: -2+119-1+6=122 ATP.
The overall equation for β-oxidation of FAs with 17 C atoms:
C 16 H 33 CO-CoA + 7 FAD + 7 NAD + + 7 HSKoA → 7 CH 3 -CO-KoA + 1 C 2 H 5 -CO-KoA + 7 FADH 2 + 7 NADH 2
Energy balance of oxidation of unsaturated fatty acids with an even number of carbon atoms
About half of the FAs in the human body are unsaturated. β-oxidation of these acids proceeds in the usual way until the double bond is between C atoms 3 and 4. The enzyme enoyl-CoA isomerase then moves the double bond from position 3-4 to position 2-3 and changes the cis conformation of the double bond to trans, which is necessary for β-oxidation. In this β-oxidation cycle, since the double bond is already present in the FA, the first dehydrogenation reaction does not occur and FADH 2 is not formed. Further, β-oxidation cycles continue, no different from the usual path.
The energy balance is calculated in the same way as for saturated FAs with an even number of C atoms, only for each double bond 1 FADN 2 and, accordingly, 2 ATP are missing.
The overall equation for β-oxidation of palmitoleyl-CoA is:
C 15 H 29 CO-CoA + 6 FAD + 7 NAD + + 7 HSKoA → 8 CH 3 -CO-KoA + 6 FADH 2 + 7 NADH 2
Energy balance of β-oxidation of palmitoleic acid: -2+8*12+6*2+7*3=127 ATP.
Hunger, physical activity → glucagon, adrenaline → TG lipolysis in adipocytes → FA in the blood → β-oxidation under aerobic conditions in muscles, liver → 1) ATP; 2) ATP, NADH 2, Acetyl-CoA, (FA) → ↓ glycolysis → glucose savings necessary for nervous tissue, red blood cells, etc.
Food → insulin → glycolysis → Acetyl-CoA → synthesis of malonyl-CoA and FA
Synthesis of malonyl-CoA → malonyl-CoA → ↓ carnitine acyltransferase I in the liver → ↓ transport of FAs into the mitochondrial matrix → ↓ FAs in the matrix → ↓ β-oxidation of FAs
Biosynthesis of IVFA. Structure of the palmitate synthase complex. Chemistry and regulation of the process.
Palmitic acid synthesis
Formation of malonyl-CoA
The first reaction in FA synthesis is the conversion of acetyl-CoA to malonyl-CoA. This regulatory reaction in FA synthesis is catalyzed by acetyl-CoA carboxylase.
Acetyl-CoA carboxylase consists of several subunits containing biotin.
The reaction occurs in 2 stages:
1) CO 2 + biotin + ATP → biotin-COOH + ADP + Fn
2) acetyl-CoA + biotin-COOH → malonyl-CoA + biotin
Acetyl-CoA carboxylase is regulated in several ways:
3) Association/dissociation of enzyme subunit complexes. In its inactive form, acetyl-CoA carboxylase is a complex consisting of 4 subunits. Citrate stimulates the union of complexes, as a result of which enzyme activity increases. Palmitoyl-CoA causes dissociation of complexes and a decrease in enzyme activity;
2) Phosphorylation/dephosphorylation of acetyl-CoA carboxylase. Glucagon or adrenaline, through the adenylate cyclase system, stimulates phosphorylation of the subunits of acetyl-CoA carboxylase, which leads to its inactivation. Insulin activates phosphoprotein phosphatase, acetyl-CoA carboxylase is dephosphorylated. Then, under the influence of citrate, polymerization of the enzyme protomers occurs, and it becomes active;
3) Long-term consumption of foods rich in carbohydrates and poor in lipids leads to an increase in the secretion of insulin, which induces the synthesis of acetyl-CoA carboxylase, palmitate synthase, citrate lyase, isocitrate dehydrogenase and accelerates the synthesis of FA and TG. Fasting or eating a diet rich in fat leads to a decrease in the synthesis of enzymes and, accordingly, FA and TG.
Formation of palmitic acid
After the formation of malonyl-CoA, the synthesis of palmitic acid continues in the multienzyme complex - fatty acid synthase (palmitoyl synthetase) .
Palmitoyl synthase is a dimer consisting of two identical polypeptide chains. Each chain has 7 active sites and an acyl transfer protein (ACP). Each chain has 2 SH groups: one SH group belongs to cysteine, the other belongs to the phosphopanthetheic acid residue. The cysteine SH group of one monomer is located next to the 4-phosphopantetheinate SH group of the other protomer. Thus, the protomers of the enzyme are arranged “head to tail”. Although each monomer contains all the catalytic sites, a complex of 2 protomers is functionally active. Therefore, 2 LCs are actually synthesized simultaneously.
This complex sequentially extends the FA radical by 2 C atoms, the donor of which is malonyl-CoA.
Palmitic acid synthesis reactions
1) Transfer of acetyl from CoA to the SH group of cysteine by the acetyltransacylase center;
2) Transfer of malonyl from CoA to the SH group of ACP by the malonyl transacylase center;
3) At the ketoacyl synthase center, the acetyl group condenses with the malonyl group to form a ketoacyl and release CO 2 .
4) Ketoacyl is reduced by ketoacyl reductase to hydroxyacyl;
5) Oxyacyl is dehydrated by hydratase into enoyl;
6) Enoyl is reduced by enoyl reductase to acyl.
As a result of the first cycle of reactions, an acyl with 4 C atoms (butyryl) is formed. Next, butyryl is transferred from position 2 to position 1 (where acetyl was located at the beginning of the first cycle of reactions). Butyryl then undergoes the same transformations and is extended by 2 C atoms (from malonyl-CoA).
Similar cycles of reactions are repeated until a palmitic acid radical is formed, which, under the action of the thioesterase center, is hydrolytically separated from the enzyme complex, turning into free palmitic acid.
The overall equation for the synthesis of palmitic acid from acetyl-CoA and malonyl-CoA is as follows:
CH 3 -CO-SKoA + 7 HOOC-CH 2 -CO-SKoA + 14 NADPH 2 → C 15 H 31 COOH + 7 CO 2 + 6
H 2 O + 8 HSKoA + 14 NADP +
Synthesis of FAs from palmitic and other FAs
Elongation of FAs in elongase reactions
Lengthening of the fatty acid is called elongation. FAs can be synthesized as a result of elongation of palmitic acid and other longer FAs in the ER. There are elongases for each LC length. The sequence of reactions is similar to the synthesis of palmitic acid, but in this case the synthesis occurs not with ACP, but with CoA. The main elongation product in the liver is stearic acid. In nerve tissues, long-chain FAs (C = 20-24) are formed, which are necessary for the synthesis of sphingolipids.
Synthesis of unsaturated FAs in desaturase reactions
The incorporation of double bonds into FA radicals is called desaturation. Desaturation of FAs occurs in the ER in monooxygenase reactions catalyzed by desaturases.
Stearoyl-CoA desaturase– integral enzyme, contains non-heme iron. Catalyzes the formation of 1 double bond between 9 and 10 carbon atoms in FA. Stearoyl-CoA desaturase transfers electrons from cytochrome b 5 to 1 oxygen atom, with the participation of protons this oxygen forms water. The second oxygen atom is incorporated into stearic acid to form its hydroxyacyl, which dehydrogenates to oleic acid.
FA desaturases present in the human body cannot form double bonds in FAs distal to the ninth carbon atom, therefore FAs of the ω-3 and ω-6 families are not synthesized in the body, are essential and must be supplied with food, as they perform important regulatory functions . The main FAs formed in the human body as a result of desaturation are palmitoleic and oleic.
Synthesis of α-hydroxy FAs
Synthesis of other FAs, α-hydroxy acids, also occurs in nervous tissue. Mixed-function oxidases hydroxylate C22 and C24 acids to form cerebronic acid, found only in brain lipids.
Carbohydrates make up the bulk of the human diet and provide a significant portion of the body's energy needs. With a balanced diet, the daily amount of carbohydrates is on average 4 times higher than the amount of proteins and fats.
The role of carbohydrates in nutrition:
1. Carbohydrates do energy function. When 1 g of carbohydrates is oxidized, 4.1 kcal of energy is released. Glucose, into which the bulk of carbohydrates is broken down, is the main energy substrate in the body.
2. Muscle activity accompanied by significant glucose consumption. During physical work, carbohydrates are consumed first, and only when their reserves (glycogen) are depleted are fats included in the exchange.
3. Carbohydrates are essential for normal function central nervous system, whose cells are very sensitive to a lack of glucose in the blood.
4. Carbohydrates do structural function. Simple carbohydrates serve as a source of formation of glycoproteins, which form the basis of connective tissue.
5. Carbohydrates are involved in the metabolism of proteins and fats. Fats can be formed from carbohydrates.
6. Carbohydrates of plant origin (cellulose, pectin substances) stimulate intestinal motility and promote the elimination of toxic products that accumulate in it.
Sources carbohydrates serve predominantly plant products, especially flour products, cereals, sweets. In most foods, carbohydrates are presented in the form of starch and, to a lesser extent, in the form of disaccharides (milk, sugar beets, fruits and berries). For better absorption of carbohydrates, it is necessary that most of them enter the body in the form of starch.
Starch is gradually broken down in the gastrointestinal tract into glucose, which enters the blood in small portions, which improves its utilization and maintains a constant blood sugar level. When large amounts of sugar are administered at once, the concentration of glucose in the blood increases sharply, and it begins to be excreted in the urine. The most favorable conditions are considered when 64% of carbohydrates are consumed in the form of starch, and 36% in the form of sugars.
Consumption rate carbohydrates depends on the intensity of work. During physical work, carbohydrates are required in larger quantities. On average, per 1 kg of body weight is required 4-6-8 g carbohydrates per day, i.e. approximately 4 times more than proteins and fats.
Excess carbohydrate intake can lead to obesity and excessive overload of the gastrointestinal tract, because plant foods rich in carbohydrates are usually more voluminous, cause a feeling of heaviness, and impair the overall digestibility of food.
Lack of carbohydrates in food is also undesirable due to the risk of developing hypoglycemic conditions. Carbohydrate deficiency, as a rule, is accompanied by general weakness, drowsiness, decreased memory, mental and physical performance, headache, decreased digestibility of proteins, vitamins, acidosis, etc. In this regard, the amount of carbohydrates in the daily diet should not be less than 300 g
Closely related to the group of carbohydrates are substances found in most plant foods that are poorly digestible by the human body - pectin substances (indigestible carbohydrates) and fiber.
Pectic substances are vegetable gelling substances with high sorption (absorbing) ability. They have a beneficial effect in the treatment of diseases of the digestive system, burns and ulcers, and also have the ability to neutralize some toxic substances (they are especially active in removing heavy metal salts, such as lead compounds, from the body).
There are a lot of pectin substances in oranges, apples, black currants and other fruits and berries.
Cellulose(other names - coarse vegetable, or indigestible, or food, or dietary fiber) is a polysaccharide that is part of the massive cell walls of plant foods. It has a fibrous, rather coarse structure.
Common sources of dietary fiber are bran, bread, and cereals (especially buckwheat and oatmeal). Large quantities are found in many vegetables, fruits, leaves and stems of plants; there is especially a lot of it in the shells of grains and in the skins of fruits. When canning vegetables and fruits, dietary fiber is completely preserved (except for juices without pulp).
Without having a high calorie content, most vegetables and fruits, however, due to their high content of indigestible carbohydrates, contribute to a quick and fairly persistent feeling of satiety: since dietary fiber has the ability to absorb a lot of liquid, they swell in the stomach, fill part of its volume - and as a result saturation occurs faster. The fibers themselves do not carry a single calorie into the body.
The value of fibers lies in the fact that, being a fairly voluminous component of daily nutrition, they are not digested by the human body. The presence of a large amount of fiber somewhat reduces the overall digestibility of food. However, its complete absence has a detrimental effect on the functioning of the gastrointestinal tract.
Fiber causes proper peristalsis (movement of the walls) of the intestine and thereby promotes the movement of food through the digestive canal and the removal of undigested nutrients from the body.
The required amount of fiber in food is ensured by the correct combination of animal and plant products in the daily diet.
After breakdown, fiber, like other polysaccharides, turns into sugars. However, there are no enzymes in the human digestive tract that could carry out such a breakdown. Only a small part of it can be digested under the influence of microorganisms in the intestines, but the bulk is removed from the body without changes. Thanks to this external uselessness, fiber and pectins are called ballast substances.
Ballast substances also perform an important function in the digestion process: fibers are fermented by intestinal bacteria and literally help to grind food; by irritating the nerve endings of the intestinal walls, they increase peristalsis. If food is poor in ballast substances, intestinal motility is disrupted, therefore, to avoid these disorders, it is recommended to use roughage foods rich in fiber.
In addition, dietary fiber has the ability to stimulate metabolism, since fiber prevents the absorption of toxins that come with food or are formed during its processing, and serve as a kind of whisk: moving along the digestive tract, they take with them everything that has stuck to the walls and remove from the body.
Another advantage of dietary fiber is that it has the ability to reduce the level of endogenous cholesterol (this is cholesterol that does not enter us with food, but is produced by the body itself in the liver from bile acids that enter the liver from the intestines).
Hemicellulose: like fiber, or cellulose, it is part of the cell walls of grain products, and small quantities are found in the pulp of fruits and vegetables. It is able to retain water and bind metals.
Oxidation of fatty acids (beta oxidation). Role H.S. – Ko in this process. Energy of complete oxidation of steoric acid to CO 2 c H 2 O . Calculate the number of ATP molecules formed during oxidation.
FA activation occurs in the cytoplasm, and beta-oxidation occurs in mitochondria.
Acyl-CoA cannot pass through the mitochondrial membrane. Therefore, there is a special mechanism for the transport of FAs from the cytoplasm into the mitochondrion with the participation of the substance “carnitine”. In the inner membrane of mitochondria there is a special transport protein that ensures transfer. Thanks to this, acylcarnitine easily penetrates the mitochondrial membrane.
Cytoplasmic and mitochondrial carnitine acyltransferases are different in structure, and they also differ from each other in kinetic characteristics. The Vmax of cytoplasmic acylcarnitine transferase is lower than the Vmax of the mitochondrial enzyme, and also lower than the Vmax of β-oxidation enzymes. Therefore, cytoplasmic acylcarnitine transferase is a key enzyme in the breakdown of fatty acids.
If a fatty acid enters the mitochondria, it will necessarily undergo catabolism to acetyl-CoA.
The most compact “fuel” that satisfies the body’s energy needs is fatty acids, which is determined by the characteristics of their chemical structure. Per 1 mole, complete oxidation of fatty acids releases several times more usable chemical energy than oxidation of carbohydrates; for example, the oxidation of 1 mol of palmitic acid produces 130 mol of ATP, while the oxidation of 1 mol of glucose produces 38 mol of ATP. Per unit weight, the energy output also differs by more than two times (9 kcal per 1 g of fat versus 4 kcal per 1 g of carbohydrates or proteins). This high energy yield is based on the same reason that makes gasoline, petroleum and other petroleum products such effective fuels for generating thermal and mechanical energy, namely the high degree of reduction of carbon in long alkyl chains. The main part of the fatty acid molecule consists of repeating units (CH2)n, i.e., a structure maximally enriched in hydrogen. As we saw from the previous presentation, the energy stored during biological oxidative processes is formed mainly in connection with the controlled transfer of electrons from the hydrogen atoms of the respiratory chain, coupled with the phosphorylation of ADP to ATP. Since fatty acids are composed primarily of carbon and hydrogen and thus contain significantly fewer oxygen atoms than carbohydrates, the oxidation of fatty acids is accompanied by the absorption of proportionately more oxygen and, therefore, the formation of more ATP during oxidative phosphorylation.
It has been established that the oxidation of fatty acids occurs most intensively in the liver, kidneys, skeletal and cardiac muscles, and in adipose tissue. In brain tissue, the rate of fatty acid oxidation is very low, because The main source of energy in brain tissue is glucose.
β-Oxidation is a specific pathway of fatty acid catabolism, in which 2 carbon atoms are sequentially separated from the carboxyl end of a fatty acid in the form of acetyl-CoA. The metabolic pathway - β-oxidation - is so named because fatty acid oxidation reactions occur at the β-carbon atom. The reactions of β-oxidation and subsequent oxidation of acetyl-CoA in the TCA cycle serve as one of the main energy sources for ATP synthesis via the oxidative phosphorylation mechanism. β-Oxidation of fatty acids occurs only under aerobic conditions.
Activation of fatty acids
Before entering into various reactions, fatty acids must be activated, i.e. are connected by a macroergic bond with coenzyme A:
RCOOH + HSKoA + ATP → RCO ~ CoA + AMP + PPi.
The reaction is catalyzed by the enzyme acyl-CoA synthetase. The pyrophosphate released during the reaction is hydrolyzed by the enzyme pyrophosphatase: H 4 P 2 O 7 + H 2 O → 2 H 3 PO 4.
The release of energy during hydrolysis of the high-energy bond of pyrophosphate shifts the equilibrium of the reaction to the right and ensures the completeness of the activation reaction.
Acyl-CoA synthetase are found both in the cytosol and in the mitochondrial matrix. These enzymes differ in their specificity for fatty acids with different hydrocarbon chain lengths. Fatty acids with short and medium chain length (from 4 to 12 carbon atoms) can penetrate into the mitochondrial matrix by diffusion. Activation of these fatty acids occurs in the mitochondrial matrix. Long-chain fatty acids, which predominate in the human body (12 to 20 carbon atoms), are activated by acyl-CoA synthetases located on the outer membrane of mitochondria.
The breakdown of activated fatty acids occurs in accordance with the hypothesis b - oxidation F. Knoop, proposed in 1904 b - oxidation occurs inside mitochondria
β- Fatty acid oxidation- a specific pathway of fatty acid catabolism, occurring in the mitochondrial matrix only under aerobic conditions and ending with the formation of acetyl-CoA. Hydrogen from β-oxidation reactions enters the CPE, and acetyl-CoA is oxidized in the citrate cycle, which also supplies hydrogen to the CPE. Therefore, β-oxidation of fatty acids is the most important metabolic pathway providing ATP synthesis in the respiratory chain.
β-Oxidation begins with the dehydrogenation of acyl-CoA by FAD-dependent acyl-CoA dehydrogenase, forming a double bond between the α and β carbon atoms in the reaction product, enoyl-CoA. The coenzyme FADH 2, restored in this reaction, transfers hydrogen atoms in the CPE to coenzyme Q. As a result, 2 ATP molecules are synthesized (Fig. 8-27). In the following p-oxidation reaction, a water molecule is added at the site of the double bond so that the OH group is located at the β-carbon atom of the acyl, forming β-hydroxyacyl-CoA. β-hydroxyacyl-CoA is then oxidized by NAD+-dependent dehydrogenase. Reduced NADH, oxidized in CPE, provides energy for the synthesis of 3 ATP molecules. The resulting β-ketoacyl-CoA undergoes thiolytic cleavage by the enzyme thiolase, since at the site of the cleavage of the C-C bond, a molecule of coenzyme A is added through a sulfur atom. As a result of this sequence of 4 reactions, a two-carbon residue, acetyl-CoA, is separated from the acyl-CoA. A fatty acid shortened by 2 carbon atoms again undergoes the reactions of dehydrogenation, hydration, dehydrogenation, and elimination of acetyl-CoA. This sequence of reactions is usually called the "β-oxidation cycle", meaning that the same reactions are repeated with the fatty acid radical until all the acid is converted to acetyl residues.
β -Oxidation of fatty acids.
The b-oxidation process is cyclic. For each revolution of the cycle, 2 carbon atoms are split off from the fatty acid in the form of an acetyl residue.
After this, acyl-CoA, shortened by 2 carbon atoms, again undergoes oxidation (enters a new cycle of b-oxidation reactions). The resulting Acetyl-CoA can further enter the tricarboxylic acid cycle. You need to be able to calculate the energy yield from the breakdown of fatty acids. The presented formula is true for any saturated fatty acid containing n carbon atoms. The breakdown of unsaturated fatty acids produces less ATP. Each double bond in a fatty acid means the loss of 2 ATP molecules. b-oxidation occurs most intensely in muscle tissue, kidneys, and liver. As a result of b-oxidation of FA, Acetyl-CoA is formed. The rate of oxidation is determined by the rate of lipolysis processes. Acceleration of lipolysis is characteristic of a state of carbohydrate starvation and intense muscle work. Acceleration of b-oxidation is observed in many tissues, including the liver. The liver produces more Acetyl-CoA than it needs. The liver is an “altruistic organ” and therefore the liver sends glucose to other tissues.
The liver strives to send its own Acetyl-CoA to other tissues, but cannot, since cell membranes are impermeable to Acetyl-CoA. Therefore, special substances called “ketone bodies” are synthesized in the liver from Acetyl-CoA. Ketone bodies are a special transport form of acetyl-CoA.
The fatty acid molecule is broken down into mitochondria by the gradual elimination of two-carbon fragments in the form of acetyl coenzyme A (acetyl-CoA).
C17H35COOH + 26 O2 = 18 CO2 + 18 H2O.
When stearic acid is oxidized, the cell will receive 146 ATP molecules.
To convert the energy contained in fatty acids into the energy of ATP bonds, there is a metabolic pathway for the oxidation of fatty acids to CO 2 and water, which is closely related to the tricarboxylic acid cycle and the respiratory chain. This path is called β-oxidation, because oxidation of the 3rd carbon atom of the fatty acid (β-position) into a carboxyl group occurs, and at the same time the acetyl group, including C 1 and C 2 of the original fatty acid, is cleaved from the acid.
Elementary diagram of β-oxidation
β-oxidation reactions occur in mitochondria most cells in the body (except nerve cells). Fatty acids that enter the cytosol from the blood or appear during lipolysis of their own intracellular TAGs are used for oxidation. The overall equation for the oxidation of palmitic acid is as follows:
Palmitoyl-SCoA + 7FAD + 7NAD + + 7H 2 O + 7HS-KoA → 8Acetyl-SCoA + 7FADH 2 + 7NADH
Stages of fatty acid oxidation
1. Before penetrating into the mitochondrial matrix and oxidizing, the fatty acid must activate in the cytosol. This is accomplished by the addition of coenzyme A to it to form acyl-SCoA. Acyl-SCoA is a high-energy compound. Irreversibility of the reaction is achieved by hydrolysis of diphosphate into two molecules of phosphoric acid.
Acyl-SCoA synthetases are found in the endoplasmic reticulum, on the outer membrane of mitochondria and within them. There is a wide range of synthetases specific for different fatty acids.
Fatty acid activation reaction
2. Acyl-SCoA is not able to pass through the mitochondrial membrane, so there is a way to transport it in combination with a vitamin-like substance carnitine. There is an enzyme on the outer membrane of mitochondria carnitine acyltransferase I.

Carnitine-dependent transport of fatty acids into the mitochondrion
Carnitine is synthesized in the liver and kidneys and then transported to other organs. In intrauterine period and in early years In life, the importance of carnitine for the body is extremely great. Energy supply to the nervous system children's the body and, in particular, the brain is carried out due to two parallel processes: carnitine-dependent oxidation of fatty acids and aerobic oxidation of glucose. Carnitine is necessary for the growth of the brain and spinal cord, for the interaction of all parts of the nervous system responsible for movement and muscle interaction. There are studies linking carnitine deficiency cerebral palsy and phenomenon" death in the cradle".
Young children, premature babies and low birth weight children are especially sensitive to carnitine deficiency. Their endogenous reserves are quickly depleted under various stressful situations (infectious diseases, gastrointestinal disorders, feeding disorders). Carnitine biosynthesis is sharply limited due to low muscle mass, and intake from regular foods is unable to maintain sufficient levels in the blood and tissues.
3. After binding to carnitine, the fatty acid is transported across the membrane by translocase. Here, on the inner side of the membrane, the enzyme carnitine acyltransferase II again forms acyl-SCoA, which enters the β-oxidation pathway.
4. The process itself β-oxidation consists of 4 reactions repeated cyclically. They happen sequentially oxidation(acyl-SCoA dehydrogenase), hydration(enoyl-SCoA hydratase) and again oxidation 3rd carbon atom (hydroxyacyl-SCoA dehydrogenase). In the last, transferase reaction, acetyl-SCoA is cleaved from the fatty acid. HS-CoA is added to the remaining (shortened by two carbons) fatty acid, and it returns to the first reaction. This is repeated until the last cycle produces two acetyl-SCoAs.

Sequence of reactions of β-oxidation of fatty acids
Calculation of the energy balance of β-oxidation
Previously, when calculating the oxidation efficiency, the P/O coefficient for NADH was taken equal to 3.0, for FADH 2 – 2.0.
According to modern data, the value of the P/O coefficient for NADH corresponds to 2.5, for FADH 2 – 1.5.
When calculating the amount of ATP formed during β-oxidation of fatty acids, it is necessary to take into account:
- the amount of acetyl-SCoA formed is determined by the usual division of the number of carbon atoms in the fatty acid by 2.
- number β-oxidation cycles. The number of β-oxidation cycles is easy to determine based on the concept of a fatty acid as a chain of two-carbon units. The number of breaks between units corresponds to the number of β-oxidation cycles. The same value can be calculated using the formula (n/2 -1), where n is the number of carbon atoms in the acid.
- number of double bonds in a fatty acid. In the first β-oxidation reaction, a double bond is formed with the participation of FAD. If a double bond is already present in the fatty acid, then there is no need for this reaction and FADN 2 is not formed. The number of lost FADN 2 corresponds to the number of double bonds. The remaining reactions of the cycle proceed without changes.
- the amount of ATP energy spent on activation (always corresponds to two high-energy bonds).
Example. Oxidation of palmitic acid
- since there are 16 carbon atoms, β-oxidation produces 8 acetyl-SCoA molecules. The latter enters the TCA cycle; when it is oxidized in one turn of the cycle, 3 molecules of NADH (7.5 ATP), 1 molecule of FADH 2 (1.5 ATP) and 1 molecule of GTP are formed, which is equivalent to 10 molecules of ATP. So, 8 molecules of acetyl-SCoA will provide the formation of 8 × 10 = 80 ATP molecules.
- for palmitic acid the number of β-oxidation cycles is 7. In each cycle, 1 molecule of FADH 2 (1.5 ATP) and 1 molecule of NADH (2.5 ATP) are produced. Entering the respiratory chain, in total they “give” 4 ATP molecules. Thus, in 7 cycles 7 × 4 = 28 ATP molecules are formed.
- double bonds in palmitic acid No.
- 1 molecule of ATP is used to activate the fatty acid, which, however, is hydrolyzed to AMP, that is, it is spent 2 macroergic connections or two ATP.
Thus, summing up, we get 80+28-2 =106 ATP molecules are formed during the oxidation of palmitic acid.
- Uniform rotation of the vessel around the vertical axis
- Tsyplenkov, Denis Ivanovich Secrets of pumping up arms from D Tsyplenkov
- Which country is the birthplace of processed cheese?
- How much does a Snickers bar weigh?
- How many degrees should vodka be?
- Types, times and methods of cooking pasta
- Seafood for your health
- How long to cook turkey fillet?
- Beef goulash with gravy How to stew beef goulash
- Bake a cupcake at home in the oven - a simple recipe
- Five rules for preparing the perfect chicken liver Marinade for chicken liver kebab
- All about one-rep max (1RM) Determine one-rep max
- Energy balance of oxidation of saturated fatty acids with an even number of carbon atoms
- List of used literature Biochemistry of muscle activity
- Biochemistry of muscle activity
- Goji berries: benefits, harm, how to take
- From slums to sports! Sergei Mironov. Athlete Sergei Mironov (bodybuilding): biography, parameters, career Sergei Mironov fitness model biography
- Video review! Mikhail Prygunov. Interview with Mikhail Prygunov What discipline did you do in athletics?
- Determination of maximum oxygen consumption
- Throwing a ball at a distance