Influenza virus replication occurs in. How does the influenza virus work? Identification of influenza viruses using RTG
They are intracellular obligate parasites, meaning that they cannot replicate or pass on their genes without help. A single viral particle (virion) is itself inert. When a virus infects a cell, it uses enzymes and the bulk of the cell's structure to replicate.
Unlike what we see in cell division processes such as and, virus replication produces many progeny that destroy the host cell and then infect other cells in the body.
Viral genetic material
Viruses can contain single-stranded/double-stranded DNA or RNA. The type of genetic material found in a particular virus depends on its nature and function. The exact nature of what happens after a host is infected varies depending on the nature of the virus.
The replication process will be different for dsDNA, ssDNA, dsRNA, and single-stranded RNA viruses. For example, double-stranded DNA viruses usually must enter host cells before they can replicate. However, single-stranded RNA viruses replicate primarily into host cells.
Once a virus infects a host, components of the viral progeny are produced by cellular machinery, and assembly of the viral capsid is a non-enzymatic process. Viruses can usually only infect a limited number of hosts. The "lock and key" mechanism is the most common explanation for this phenomenon. Certain proteins on the virus particle must match certain receptor proteins on the cell surface of a particular host.
How do viruses infect cells?
The basic process of infection and replication of the virus occurs in 6 stages:
- Adsorption - the virus binds to the host cell.
- Entry - the virus introduces its genome into the host cell.
- Viral genome replication - The viral genome is replicated using the host's cellular structure.
- Assembly - viral components and enzymes are formed and begin to assemble.
- Maturation - viruses develop from the assembled components.
- Exit - new viruses break out of the host cell in search of new victims to infect.
Viruses can infect any type of cell, including
The family of orthomyxoviruses (Greek orthos - correct, tukha - mucus) includes influenza viruses types A, B, C, which, like paramyxoviruses, have an affinity for mucin. Influenza A viruses infect humans and some species of animals (horses, pigs, etc.) and birds. Influenza viruses types B and C are pathogenic only for humans. The first human influenza virus was isolated from humans in 1933 by W. Smith, C. Andrews and P. Ladow (WS strain) by infecting white ferrets. Later, this virus was classified as type A. In 1940, T. Francis and T. Megill discovered the influenza virus type B, and in 1949, R. Taylor discovered the influenza virus type C. When classifying influenza viruses, there have always been certain difficulties associated with with their antigenic variability. Influenza viruses are divided into three types A, B and C. Type A includes several subtypes that differ from each other in their antigens - hemagglutinin and neuraminidase. According to the WHO classification (1980), human and animal influenza viruses type A are divided into 13 antigenic subtypes based on hemagglutinin (H1-H13) and 10 based on neuraminidase (N1-N10). Of these, human influenza viruses type A include three hemagglutinins (HI, H2 and NZ) and two neuraminidases (N1 and N2). For the type A virus, the subtype of hemagglutinin and neuraminidase is indicated in parentheses. For example, influenza A virus: Khabarovsk/90/77 (H1N1).Structure and chemical composition
The influenza virus has a spherical shape, with a diameter of 80-120 nm. Thread-like forms are less common. The nucleocapsid of helical symmetry is a ribonucleoprotein (RNP) strand arranged in a double helix that forms the core of the virion. RNA polymerase and endonucleases (P1 and P3) are associated with it. The core is surrounded by a membrane consisting of the M protein, which connects the RNP with a lipid bilayer of the outer shell and styloid processes consisting of hemagglutinin and neuraminidase. Virions contain about 1% RNA, 70% protein, 24% lipids and 5% carbohydrates. Lipids and carbohydrates are part of the lipoproteins and glycoproteins of the outer shell and are of cellular origin. The genome of the virus is represented by a minus-strand fragmented RNA molecule. Influenza viruses types A and B have 8 RNA fragments. Of these, 5 encode one protein, and the last 3 encode two proteins each.Antigens
Influenza viruses A, B and C differ from each other in the type-specific antigen associated with RNP (NP protein) and the M-matrix protein, which stabilizes the structure of the virion. These antigens are detected in RSC. The narrower specificity of the type A virus is determined by two other surface antigens - hemagglutinin H and neuraminidase N, designated by serial numbers. Hemagglutinin is a complex glycoprotein with protective properties. It induces in the body the formation of virus-neutralizing antibodies - antihemagglutinins, detected in the RTGA. The variability of hemagglutinin (H-antigen) determines the antigenic drift and shift of the influenza virus. Antigenic drift refers to minor changes in the H-antigen caused by point mutations in the gene that controls its formation. Such changes can accumulate in offspring under the influence of selective factors such as antibodies. This ultimately leads to a quantitative shift, expressed in a change in the antigenic properties of hemagglutinin. With antigenic shift, a complete replacement of the gene occurs, which may be based on recombination between two viruses. This leads to a change in the subtype of hemagglutinin or neuraminidase, and sometimes both antigens, and the emergence of fundamentally new antigenic variants of the virus, causing major epidemics and pandemics. Hemagglutinin is also a receptor through which the virus is adsorbed on sensitive cells, including erythrocytes, causing them to stick together , and is involved in the hemolysis of red blood cells. Viral neuraminidase is an enzyme that catalyzes the cleavage of sialic acid from the substrate. It has antigenic properties and at the same time participates in the release of virions from the host cell. Neuraminidase, like hemagglutinin, changes as a result of antigenic drift and shift.Cultivation and reproduction
Influenza viruses are cultivated in chicken embryos and in cell cultures. The optimal environment is chicken embryos, in the amniotic and allantoic cavities of which the virus reproduces within 36-48 hours. The most sensitive to the influenza virus are primary cultures of human embryonic kidney cells and some animals. Reproduction of the virus in these cultures is accompanied by a mild CPE, reminiscent of spontaneous cell degeneration. Influenza viruses are adsorbed on glycoprotein receptors of epithelial cells, into which they penetrate through receptor endocytosis. Transcription and replication of the viral genome occurs in the cell nucleus. In this case, the read individual RNA fragments in the form of m-RNA are translated into ribosomes, where the synthesis of virus-specific proteins occurs. After replication of the viral genome, a pool of viral RNAs is formed, which is used in the assembly of new nucleocapsids.Pathogenesis
Primary reproduction of the virus occurs in the epithelial cells of the respiratory tract. Through the eroded surface of the mucous membrane, the virus enters the blood, causing viremia. The circulation of the virus in the blood is accompanied by damage to the endothelial cells of the blood capillaries, resulting in an increase in their permeability. In severe cases, hemorrhages are observed in the lungs, heart muscle and other internal organs. Influenza viruses, entering the lymph nodes, damage lymphocytes, resulting in acquired immunodeficiency, which contributes to the occurrence of secondary bacterial infections. With influenza, intoxication of the body of varying severity occurs.Immunity
The mechanism of anti-influenza immunity is associated with natural factors of antiviral nonspecific protection, mainly with the production of interferon and natural killer cells. Specific immunity is provided by factors of cellular and humoral response. The first are represented by macrophages and T-killers. The second are immunoglobulins, primarily antihemagglutinins and antineurominidase antibodies, which have virus-neutralizing properties. The latter, unlike antihemagglutinins, only partially neutralize the influenza virus, preventing its spread. Complement-fixing antibodies to the viral nucleoprotein do not have protective properties even after 1.5 months. disappear from the blood of convalescents. Antibodies are detected in the blood serum 3-4 days after the onset of the disease and reach maximum titers after 2-3 weeks. The duration of specific immunity acquired after influenza infection, contrary to previous beliefs, is measured in several decades. This conclusion was reached based on a study of the age structure of the incidence of influenza caused by the A (H1N1) virus in 1977. It was found that this virus, which had been absent since 1957, affected only people under 20 years of age in 1977. Thus, after suffering an influenza infection caused by the influenza virus type A, intense immunity is formed, strictly specific to the subtype of the virus (by H- and N-antigens) that caused its formation. In addition, newborns have passive immunity due to IgG antibodies to the corresponding virus subtype A. Immunity lasts for 6-8 months.Epidemiology
The source of infection is sick people and virus carriers. Transmission of the pathogen occurs by airborne droplets. Influenza is an epidemic infection that occurs more often in the winter and winter-spring months. Approximately every ten years, influenza epidemics become pandemics, affecting the population of different continents. This is explained by the change in the H- and N-antigens of the type A virus associated with antigenic drift and shift. For example, the influenza A virus with hemagglutinin NSW1 caused the Spanish flu pandemic in 1918, which claimed 20 million human lives. In 1957, the “Asian” influenza virus (H2N2) caused a pandemic that affected more than 2 billion people. In 1968, a new pandemic variant emerged, the influenza A (H3N2) virus, called the Hong Kong virus, which continues to circulate to the present day. In 1977, it was joined by the type A virus (H1N1). This was unexpected, since an identical virus had already circulated in 1947-1957, and was then completely replaced by the “Asian” subtype. In this regard, a hypothesis arose that shift variants of the virus are not historically new. They represent serosubtypes circulating in past years. The cessation of circulation of the influenza virus, which caused the next epidemic, is explained by the collective immunity of the population that has developed to this antigenic variant of the pathogen. Against this background, there is a selection of new antigenic variants, collective immunity to which has not yet been formed. It is not yet clear where the shift antigenic variants (serosubtypes) of the influenza A virus that came out of active circulation in one or another historical period are preserved for a long time. It is possible that the reservoir for the persistence of such viruses are wild and domestic animals, especially birds, which are infected with human variants of type A influenza viruses and maintain their circulation for a long time. At the same time, genetic recombinations between avian and human viruses occur in the body of birds, which lead to the formation of new antigenic variants. According to another hypothesis, influenza viruses of all known subtypes constantly circulate among the population, but become epidemically relevant only with a decrease in collective immunity. Influenza viruses of types B and C are characterized by higher antigenic stability. Influenza B viruses cause less intense epidemics and local outbreaks. Influenza virus type C is the cause of sporadic diseases. The influenza virus is quickly destroyed by temperatures above 56°C, UV radiation, disinfectants, and detergents. It remains viable for 1 day. at room temperature, on smooth metal and plastic surfaces - up to 2 days. Influenza viruses survive at low temperatures (-70°C).Specific prevention
For the prevention of influenza, rimantadine is used, which suppresses the reproduction of the influenza virus type A. For passive prevention, human anti-influenza immunoglobulin is used, obtained from the blood serum of donors immunized with influenza vaccine. Human leukocyte interferon has a certain effect. Live and inactivated vaccines are used for vaccine prevention. When live vaccines are administered, both general and local immunity are formed. In addition, interferon induction is noted. Currently, inactivated vaccines of various types have been obtained: virion, subunit, split and mixed. Virion vaccines are produced by high-quality purification of viruses grown in chicken embryos. Subunit vaccines are purified surface antigens of the influenza virus - hemagglutinins and neuraminidase. Such vaccine preparations are characterized by reduced reactogenicity and high immunogenicity. Cleaved or disintegrated vaccines are prepared from a purified virion suspension by treatment with detergents. However, there is still no consensus on the superiority of any one of these vaccines. Inactivated vaccines induce an immune response in the system of general and local humoral immunity, but induce interferon synthesis to a lesser extent compared to live vaccines. Many years of experience in the use of live and inactivated vaccines indicate that the antigenic mismatch of vaccine strains with epidemic ones is the main, but not the only reason low effectiveness of influenza vaccine prevention. In recent years, attempts have been made to create genetically engineered and synthetic influenza vaccines.Flu
Influenza is an acute human respiratory disease that tends to spread epidemically. It is characterized by catarrhal inflammation of the upper respiratory tract, fever, and severe general intoxication. Influenza is often accompanied by severe complications - secondary bacterial pneumonia, exacerbation of chronic lung diseases. Influenza pathogens belong to the Orthomyxoviridae family. It includes three types of viruses - A, B, C. The influenza virus has a spherical shape, its size is 80-120 nm. Sometimes filamentous virions are formed. The genome is formed by a single-stranded minus-strand RNA, which consists of eight fragments, and is surrounded by a protein capsid. RNA associated with 4 internal proteins: nucleoproteins (NP) and high molecular weight proteins PI, P2, R3, involved in genome transcription and virus replication. The nucleocapsid has a helical type of symmetry. Above the capsid shell is a layer of matrix protein (M protein). On the outer, supercapsid shell, hemagglutinin (H) and neuraminidase (N) are located in the form of spines. Both glycoproteins (N and H) have pronounced antigenic properties. In influenza viruses, 13 different antigenic types of hemagglutinin (NI-13) and 10 variants of neuraminidase (N1-10) were found. Based on the internal nucleoprotein antigen, three types of influenza viruses are distinguished - A, B, C, which can be determined in RSC. Type A viruses that infect humans have three types of hemagglutinin (HI, H2, H3) and two neuraminidases (N1, N2). Depending on their combinations, variants of influenza A viruses are distinguished - H1N1, H2N2, H3N2. they are determined in the hemagglutination inhibition reaction with appropriate sera. Influenza viruses are easily cultivated in chicken embryos and various cell cultures. Maximum accumulation of viruses occurs after 2-3 days. In the external environment, the virus quickly loses its infectivity through drying out. At low temperatures in the refrigerator it is stored for a week, at -70 ° C - much longer. Heating causes it to inactivate after a few minutes. Under the influence of ether, phenol, formaldehyde, it is quickly destroyed.Virological diagnostic method
The material for research is swabs from the nasopharynx, nasal discharge, which is taken with dry or wet sterile cotton swabs in the first days of the disease, sputum. Viruses can be found in blood and cerebrospinal fluid. In case of fatal cases, pieces of the affected tissues of the upper and lower respiratory tract, brain, etc. are removed. Nasopharyngeal swabs are taken on an empty stomach. The patient should gargle three times with sterile saline sodium chloride solution (10-15 ml), which is collected in a sterile wide-necked jar. After this, wipe the back wall of the pharynx and nasal passages with a piece of sterile cotton wool, then dip it into a jar with rinsing. You can take the material with a sterile swab moistened in a sodium chloride solution, which is used to thoroughly wipe the back wall of the pharynx. After collecting the material, the swab is immersed in a test tube with physiological solution, to which 5% of inactivated animal serum is added. In the laboratory, swabs are rinsed in liquid, squeezed against the side of the tube, and removed. The drain is kept in the refrigerator to settle, then the middle part of the liquid is collected into sterile tubes. Antibiotics penicillin (200-1000 IU/ml), streptomycin (200-500 μg/ml), nystatin (100-1000 IU/ml) are added to the material to destroy accompanying microflora, kept for 30 minutes at room temperature and used to isolate viruses. having previously checked it for sterility. A sensitive method for isolating viruses that infect 10-11-day-old chicken embryos. Material in a volume of 0.1-0.2 ml is injected into the amniotic or allantois cavity. As a rule, 3-5 embryos are infected. Embryos are incubated at an optimal temperature of 33-34 ° C for 72 hours. In order to increase the number of virions in the test material, it is pre-concentrated. To do this, they use methods of adsorption of viruses on chicken red blood cells, treatment with a 0.2% trypsin solution in order to enhance the infectious properties of viruses, or precipitate them using special methods. After incubation, chicken embryos are cooled at a temperature of 4 ° C for 2-4 hours, then sucked off with sterile with pipettes or a syringe, allantoic or amniotic fluid. In this case, the presence of an infectious virus is determined using RGA. To do this, mix equal volumes (0.2 ml) of virus-resistant material and 1% suspension of chicken red blood cells. A positive reaction (the presence of a virus in the material) is indicated by the sedimentation of erythrocytes in the form of an umbrella. If there is a virus in the material that has hemagglutinous properties, it is titrated using an expanded RGA, determining the titer of hemagglutinous activity. Using this reaction, the titer of the hemagglutinating virus is determined - the highest dilution of the material that still gives the hemagglutination reaction. This amount of virus is taken as one hemagglutinous unit (HAU).Identification of influenza viruses using RTGA
To do this, first prepare a working dilution of the viral material, which contains 4 GAO of the virus in a certain volume. The reaction is taken into account after the formation of a sediment of erythrocytes in the control wells. A positive reaction is indicated by a delay in hemagglutination in the test wells. Influenza viruses can be isolated using various cell culture lines - human embryo, monkey kidneys, continuous canine kidney cell line (MDCK) and others. In cell cultures, the cytopathic effect of viruses is manifested (the appearance of cells with scalloped edges, vacuoles, the formation of intranuclear and cytoplasmic inclusions), which ends with the degeneration of the cell monolayer. To identify the isolated viruses, RTGA is used (provided that the hemagglutinin titer in the culture fluid is at least 1:8). In addition to this reaction, you can use RGGads, however, it is less sensitive and requires an immune serum titer of at least 1:160 as well as RSK, RN, REMA, etc.Serological study
Serological testing is used to confirm the diagnosis of influenza. It is based on determining a fourfold increase in the antibody titer in the patient's serum. The first serum is obtained at the onset of the disease in the acute period (2-5-1 days of illness), the second - after the 10-14th day of the disease. Since the serums can be mixed at the same time, the first of them is stored in the refrigerator at a temperature of -20 ° C. Most often, RTGA, RSK, RNGA are used. These reactions are performed with special sets of standard viral diagnostics (reference strains of influenza virus of various serological types). Since patient sera may contain nonspecific hemagglutination inhibitors, they are first heated at a temperature of 56 ° C and also treated with a special enzyme (for example, neuraminidase) or solutions of potassium periodate, rivanol, manganese chloride, white tire suspension, etc. according to special schemes. ANDHemagglutination inhibition reaction
The hemagglutination inhibition reaction can be performed in test tubes (macromsh tod) or in special plates for immunological studies. The reaction is considered positive when a compact, dense sediment of red blood cells with smooth edges is formed.Express diagnostics
The method is based on identifying specific viral antigens in the test material using immunofluorescence in direct or indirect RIF. Mucus is obtained from the nasal passages or the back wall of the pharynx, centrifuged, and smears are prepared on glass slides from the sediment of columnar epithelial cells of the mucous membrane. they are treated with immunofluorescent sera conjugated to fluorochromes, for example, FITC (fluorescein isothiocyanate). When examining drugs using a fluorescent microscope, a characteristic green-yellow glow of influenza viruses is observed, which are localized at the onset of the disease in the nuclei of epithelial cells. Recently, it has been proposed to use ELISA, RZNGA, and PCR to indicate specific viral antigens.Adsorption
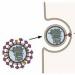
The famous “H5N1” stands for “hemagglutinin type five, neuraminidase type one” - these two proteins stick out on the surface of the influenza virus (in Fig. 1, hemagglutinin is green and neuraminidase is gray).
With the help of hemagglutinin, the influenza virus attaches to receptors on the surface of cells. The initial target of the virus is the cells of the ciliated epithelium of the respiratory tract, but this is not why we love it: hemagglutinin can attach to the receptors of many other cells, including red blood cells. If one virus attaches to two (adjacent) red blood cells at the same time, the red blood cells will stick together! Hence the name of the protein - “blood-gluing”.
rice. 1
Implementation
The stupid cell absorbs the virus that has attached itself to it by phagocytosis - like, eats it. Why, why do children always put all sorts of nasty things in their mouths?! However, the virus is still inside the cell as food, inside a phagocytotic vesicle (in Fig. 2 - “endosome”). The endosome merges with the lysosome, a digestive vacuole is formed, protons are pumped into it from the cytoplasm to create an acidic environment (this process is shown in Fig. 2) - a little more, and we will digest the virus (with the words “protein food, what’s the difference”).

rice. 2
Strip
But the virus is ready for this turn of events:
 rice. 3 |
- Hemagglutinin is modified under the influence of an acidic environment - its surface becomes hydrophilic, and it (previously attached to the receptor on the inner surface of the endosome membrane, now) is embedded inside this membrane.
- Protons pumped into the endosome pass through special channel proteins (M2 proteins, indicated in Fig. 1 and Fig. 3) through the lipid shell of the virus and reach the protein shell of the virus (in Fig. 1 - a circle of white balls - M1 proteins) . Because of this, the protein shell is destroyed (in Fig. 3, the M1 proteins of the destroyed protein shell are indicated as red stars).
- The lipid envelope of the virus (due to the penetrating action of hemagglutinin) fuses with the (lipid) membrane of the phagosome; The RNA of the virus ends up in the cytoplasm of the cell.
Virus replication
The virus RNA released into the cytoplasm is itself completely safe.
- Proteins cannot be made on it, because it is minus RNA (proteins are not encoded by it, but by the complementary plus strand, which does not yet exist).
- It is also impossible to make RNA on it - our cells generally do not have an enzyme capable of doubling RNA.
“Oh-ho-ho, you don’t have anything,” the flu virus grumbles, shaking his mustachioed head, “but it’s okay, I brought everything with me.” The virus brought with it the proteins PB1, PB2 and PA, which together form the viral RNA-dependent RNA polymerase - it can duplicate RNA. But bad luck! Any polymerase needs a primer to start working, but the forgetful flu didn’t take it with it! Everything is over?!
Calm down, don't panic! - With these words, the whole company (8 viral RNAs and 3 viral enzymes) is sent to the cell nucleus. There the flu gets full service:
- primers for viral RNA replication (to obtain plus RNA) are sections cleaved from cellular RNA;
- processing: sections that served as primers for RNA synthesis - these were caps, so the modification of the 5" end was carried out at the very beginning; at the end of the synthesis, polyadelation of the 3" end occurs;
- splicing: some viral RNA containing information for two proteins is cut into two parts.
In this way, plus RNAs are synthesized, which can serve as templates for the synthesis of viral proteins and viral minus RNAs.
Then everything is simple: the stupid cell, using its own ribosomes from its own amino acids, synthesizes the proteins of the virus, including RNA-dependent RNA polymerase. Influenza minus RNA is also vigorously produced inside the nucleus. The assembly of viral particles occurs in the cytoplasm, on the inner surface of the cell membrane. The finished virus leaves the cell by exocytosis (budding), neuraminidase bites the last thread that connects the cell and the newborn virus... A new (evil) little life comes out into the world!

rice. 4
K. SOLTISSEK and H.-D. KLENK (SN. SCHOLTISSEK, H.-D. KLENK)
I. INTRODUCTION
There are a number of reviews on the problem of influenza virus replication. The literature prior to 1968 is summarized in articles by Hoyle (1968) and Scholtissek (1969); more recent works include reviews by White (1973) and Compans and Choppin (1974).
Most of the data on replication were obtained from the study of influenza virus type A. Significant differences in the replication mechanisms of other types of influenza virus have not yet been found.
Several cell culture systems have become widespread that are convenient for studying replication, for example propagation of the WSN strain of influenza virus in MDBK cells (Choppin, 1969) or propagation of fowl plague virus (FPV) in chick embryo fibroblast cells. An example of the growth curve of the last virus in a single cycle has been proven in 30, where the latent period is approximately 3 hours and virus production reaches a plateau between 8 and 12 hours. In general, in such cell systems a high yield of infectious virus is observed, and the synthesis of cellular proteins is very effectively suppressed after infections. Therefore, such systems are very convenient for biochemical research.
II. ADSORPTION, PENETRATION, “STRIPPING” OF THE VIRUS
Infection of a cell with a virus begins with adsorption, i.e., attachment of the viral particle to the cell surface. For attachment, two complementary structures are required, namely: receptor sites on the cell surface and the viral component responsible for recognizing these receptor sites. The ability of the influenza virus to interact with erythrocytes of various origins and agglutinate them has been known for many years (Hirst, 1941; McClelland, Hare, 1941) Hemagglutination has been used as a model reaction for the interaction of influenza virus with the cell surface, and “most of your knowledge of this phenomenon comes from similar studies. However, one should be very careful in generalizations, since the structures of the surface of erythrocytes and the surface of infected cells can be completely different (see also Chapter 3).
A. ROLE OF HEMAGGLUTIININ IN ADSORPTION
The component of the virion involved in binding is the HA* “spike”. The role of viral β-proteins in the initiation of infection was studied using antibodies specific to two surface proteins: HA* and NA*. These antibodies can be obtained using recombinant viruses. For example, a cross between the AO and A2 viruses results in the formation of the recombinant X7F1, which carries HA*, AO, and NA* A2 (Kilbourne et al., 1968). Antiserum to the X7F1 virus does not inhibit NA* of influenza viruses of the AO type, but inhibits hemagglutination and neutralizes the infectivity of viruses of this type. The interaction of the same serum with influenza A2 viruses does not inhibit hemagglutination or infectivity, although the neutralization of NA* activity is complete. Thus, NA* is not involved in the process of initiating infection, and only NA* appears to be responsible for adsorption. This concept is supported by data that viral particles from which only the neuraminidase “spikes” were removed by proteolytic enzymes remained infectious (Schulze, 1970).
There is evidence that the hemagglutinating part is localized on the outer part of the HA* “spike,” which is rich in carbohydrates (see Chapter 3). Carbohydrates appear to be essential for HA* function, since non-glycosylated HA* proteins are unable to bind to red blood cells (Klenk et al., 1972b).
B. FLU VIRUS RECEPTOR
Carbohydrates are an essential component not only of hemagglutinin, but also of the viral receptor on the cell surface. Hirst (1942) observed that the virus-erythrocyte complex is unstable and that the receptor on the surface of the cell is destroyed by the virus enzyme. As was later shown, this enzyme is neuraminidase , which cleaves neurastic acid from glycoproteins (Klenk et al., 1955; Klenk, Stoffel, 1956; Gottschalk, 1957). This was the first demonstration of an enzyme that is an integral part of a viral particle. Bacterial neuraminidases are also capable of destroying influenza virus receptors (Burnet, Stone , 1947). Thus, it was found that the receptor for the influenza virus is a glycoprotein containing neuraminic acid.
Since then, a large amount of information has been accumulated
about the myxovirus receptor, summarized recently in a review
Hughes (1973). The data obtained are briefly summarized as follows:
blowing Receptor areas contain remnants of neuromi-
ioic acid, which are present in carbohydrate chains
glycoproteins. Non-oxidizing terminal residues of neuromi-
nosnla are necessary for the interaction of glycoproteins with viruses
Rus flu. Treatment with neuraminidase completely removes
binding activity. Degradation studies with is
using periodate it is assumed that for the connecting
activity requires an intact neuramine molecule
acids (Suttajit and Winzler, 1971). carboxyl group,
probably also plays an important role, since it is necessary
apparently, and electrostatic forces (Huang, 1974).
It seems likely that there is only a weak spice
physicality relative to the structure with which it is associated
neuraminic acid, since it has been shown that whole
a set of glycoproteins containing jaeuraminic acid,
binds to myxoviruses. Moreover, gangliosides (gly-
are active in this regard (Haywood, 1975).
It is hoped that the issue of influenza virus binding will become more clear once the molecular structure of the receptor is established. To some extent this has already been achieved in the case of erythrocytes (Marchesi et al., 1973). Additional information may also be provided by studying the attachment of myxoviruses to artificial membranes (Tiffany and Blough, 1971).
B. POSSIBLE MECHANISMS OF PENETRATION AND “STRIPPING”
Two different “mechanisms of penetration and “undressing” of not only the influenza virus, but also viruses in general that have an outer shell have been proposed. Both points of view are based mainly on studies in the electron microtrench. One of these mechanisms is vironexis, which is believed to is a process of ppnocytosis, when viral particles are included in pinosomes, which subsequently merge with lysosomes, and lysosome enzymes cause the “undressing” of the virus (Fazekas de St. Grot, 1948). Electron microscopic confirmation of this point of view was obtained in the works of Dales and Choppin (1962) , and Dourmashkin and Tyrrell (1970).At 10 minutes after infection, viral particles were visible in direct contact with the cell surface, and by 20 minutes particles were found inside cytoplasmic vacuoles. In contrast to this study by Morgan and Rose (1968) suggest that entry may result from fusion of the viral envelope with the host cell membrane.Therefore, there is currently no consensus regarding the mechanism of influenza virus entry.
As described in Chap. 5, influenza virus virions contain RNA polymerase associated with their ribonucleoprotein components. It is therefore unlikely that the process of “undressing” would be spatially separated from the process of ribonucleoprotein release. And this stage occurs on the cell surface, according to the mechanism of membrane fusion, and in phagocytic vesicles, according to the virohexis mechanism.
III. TRANSCRIPTION A. RNA SYNTHESIS SEQUENCE
After “undressing,” the virion’s sRNA must be transcribed into complementary RNA. The RNA polymerase introduced with the infecting particle must function in the first stage of virus propagation (see Chapter 5). The influenza virus genome can be isolated from virions only in the form
separate fragments (see Chapter 6). Moreover, it functions in the form of separate fragments, as was shown by genetic analysis (chapter 7) and stepwise inactivation of an infectious virus (Scholtissek, Rott, 1964). In this regard, it should be assumed that the polymerase initiates the synthesis RNA in each individual fragment Since RNA does not exist in the cell as a free molecule, but is always dressed with a protein, the question arises: what protein is the viral RNA associated with during its replication?
Experiments carried out to study the synthesis of RNA of the influenza virus present great difficulties, since actinomycin D cannot be used to detect the production of viral RNA when specifically suppressing the synthesis of cellular RNA, since this antibiotic suppresses the reproduction of the influenza virus (Barry et al., 1962 ; Rott, Scholtissek, 1964; Barry et al., 1965; Pons, 1967). For this reason, to determine the time sequence of RNA synthesis, specific hybridization of pulse-labeled RNA at various points after infection with an excess of either unlabeled vRNA or vcRNA was used, followed by treatment with RNase (Scholtissek and Rott, 1970). In the early stages of the infectious cycle, vcRNA synthesis prevailed, reaching a maximum approximately in the second hour after infection, whereas in the later stages, most of the virus-specific RNA produced was vRNA. Krug (1972), using a different method, also showed that 4 h after infection, BIKRNA synthesis almost completely ceases. After phenol extraction, a relatively small amount of lncRNA is detected (Scholtissek and Rott, 1970).
Due to the fact that one or another type of viral RNA has informational1!! functions and is used as a template for the synthesis of viral proteins (see section IVA), there may be some translational control at the level of differential catabolism of viral RNA. Therefore, pulse-chase experiments were performed to study the stability of influenza virus RNA in vivo. It has been established that, in contrast to cellular RNA, both types of viral RNA are completely stable during the 90-day period (Scholtissek et al., 1972).
Previously, when studying the synthesis of viral RNA in vivo, when actinomycin D was added in the late stages of the infectious cycle (Duesberg and Robinson, 1967; Nayak, 1970; Ma-hy, 1970), they did not take into account that the antibiotic specifically inhibits the synthesis of complementary RNA in vivo (Scholtissek and Rott, 1970; Pons, 1973). Since lncRNA is released from infected cells in the form of at least five separate fragments, it was concluded that viral RNA is also synthesized in the form of fragments (Pons and Hirst, 1968).
B. LOCALIZATION OF VIRAL RNA SYNTHESIS INSIDE THE HOST CELL
From the data obtained by autoradiography, it was concluded that the site of viral RNA synthesis appears to be the cell nucleus (Scholtissek et al., 1962; Barry et al., 1974). Since the pulse periods used in these studies were still too long, it cannot be excluded that viral RNA is synthesized in the cytoplasm of the cell and is then transported to the nucleus, where it can accumulate. In addition, vRNA and cRNA can be synthesized in different places in the cell.
B. INHIBITION OF VIRAL RNA SYNTHESIS 1. Actimomycin D, mithramycin and a-amanitin
When actinomycin D or mithramycin, which interfere with DNA template function, is added to infected cells at a time when viral RNA-dependent RNA polymerase is already present (for example, 2 hours after infection), vRNA continues to be synthesized for approximately 2 more hours. VcRNA production, however, immediately stops. Later, vRNA synthesis also decreases, indicating that it requires continuous formation of vRNA (Rott et al., 1965; Scholtissek and Rott, 1970; Scholtissek et al., 1970; Pons, 1973). Gregoriades (1970) showed that actinomycin D also has a strong effect on vRNA synthesis when added late in the infection cycle. In these experiments, the synthesis of viral RNA was determined by the increase in the incorporation of labeled uridine into the total RNA of infected cells. This increase can be eliminated by the addition of actinomycin D. However, it should be borne in mind that influenza virus infection causes an increase in the incorporation of labeled uridine into the cell after infection (Scholtissek et al., 1967) and that actinomycin D has an inhibitory effect on this incorporation (Scholtissek et al., 1967). ., 1969). siAmanitin, which has no affinity for DNA, influences the activity of one of the cellular RNA polymerases (RNA podimerase II), and also inhibits the synthesis of cRNA when added to the culture liquid immediately after infection (Rott and Scholtissek, 1970; Mahy et al., 1972).
The mechanism of specific inhibition of cfRNA synthesis by these antibiotics is not entirely clear, since they do not affect the formation of cfRNA in vitro. Thus, these antibiotics act only in vivo, although the enzyme that synthesizes cfRNA can be isolated from cells to which 2 h after infection, actinomycin D was added (Scholtissek and Rott, 1969a).
The reproduction of influenza viruses can also be suppressed by other effects on the host cell DNA - the introduction of mitomcin C, pretreatment with ultraviolet irradiation, or removal of cell nuclei before infection (Barry, 1964; Rott et al., 1965; Nayak, Rasmussen, 1966; Fol- Lett et al., 1974; Kelly et al., 1974). The mechanism by which these effects affect the replication of the influenza virus may be the same as the mechanism of action of other antibiotics. The only assumption that can be made from these studies is that there is a need for "functionally" active cell nuclei and (or) in the DNA-dependent function of the cell for the propagation of influenza viruses. It is impossible to say what these functions are.
2. Cycloheximide
When cycloheximide, which specifically inhibits protein synthesis in animal cells, is added 2 hours after infection with the influenza virus, the formation of vRNA immediately stops, while the formation of vRNA still continues for at least 2 hours (Scholtissek and Rott, 1970; Pons, 1973). It is not yet known whether continuous synthesis of the viral or cellular protein used as a "Quality/cofactor for the polymerase that synthesizes vRNA is required, or whether some "protein (e.g., NP protein) is required to stabilize the newly synthesized vRNA, or suppression of the synthesis of a specific viral "protein leads to the continuous formation of cRNA, the synthesis of which is normally turned off 3 hours after infection. This shutdown may be necessary to trigger vRNA synthesis. Studies with temperature-sensitive mutants should answer some of these questions.
Experiments by Bean and Simpson (1973) showed that primary transcription in vivo (synthesis of cRNA on an RNA template using the polymerase of the infecting particle) is not suppressed by cycloheximide, while actinomycin D suppresses transcription completely. Thus, cycloheximide does not affect in vivo the activity of the polymerase introduced with the infectious particle and synthesizing cfRNA; however, it suppresses the synthesis of a new polymerase necessary for the production of cfRNA.
3. Glucosamine
Glucosamine is known to deplete the UTP pool in chick embryo cells by forming UTP-]M-acetylglucosamine (Scholtissek, 1971). When Earl's solution containing glucose is used as a culture medium, it
has an effect only on the synthesis of viral glncoproteins (see section V). If, however, glucose as an energy source is replaced with lyruvate or fucose, then the depletion of the UTP pool by these amino sugars occurs approximately 10 times more actively. Under these conditions, the UTP pool of the host cell becomes a specific limiter on the rate of vRNA synthesis, while the synthesis of cellular RNA is not yet affected (Scholtissek, 1975). As a result of the suppression of viral RNA synthesis, the formation of viral proteins is also absent.
These data can be interpreted in two ways: either the viral RNA-dependent RNA polymerase has a low affinity for UTP compared to cellular DNA-dependent and RIC polymerases, or there are two more or less independent pools of UTP in the cell, one of which can " be used for viral RNA synthesis and is more affected by glucosamine than the other pool, which can be used as a substrate by RNA polymerases.
D. SYNTHESIS OF VIRAL RNA IN VITRO
In cells infected with influenza virus, several researchers discovered RNA-dependent RNA polymerase (Ho, Walters, 1966; Scholtissek, Rott, 1969a; Skehel, Burke, 1969; Ruck et al., 1969; Mahy, Bromley, 1970; Compans, Caliguiri, 1973). Most of the enzyme activity was found in the microsomal fraction of infected cells. In the in vitro system, this activity is removed by RNase, but not by DNAse. This means that the internal template is RNA- The reaction requires the presence of all four nucleoside triphosphates and is sensitive to actin-icin D. Most of the production of the in vitro reaction has a low relative molecular weight. Data from Horisberger and Guskey (1973) suggest that two different enzyme activities are present in the cytoplasm: one is Mg++-dependent and inhibited by relatively high salt concentrations, the other is Mn++-dependent and more salt-resistant. The latter enzyme activity is also found inside the viral particle (see Chapter 5).
Conflicting results have been obtained regarding the cytoplasmic enzyme product in the in vitro system. Ruck ■et al. (1969) reported that in their hands this enzyme synthesizes at least “some of the virion-type RNAs (14 to 19S). The authors came to this conclusion when determining the base composition of the product in an in vitro system after incubating the microsomal fraction with all four -labeled nucleoside triphosphates of known specific radioactivity. However, data from the study of the nearest
to adenylic acid neighbors, obtained in the same work using [(a-32P]ATP, are consistent with the nearest neighbor analysis data obtained by Scholtissek (1969), which resulted in the conclusion that the product in the in vitro system has the structure of vcRNA. Mahy and Bromley (1970) in their original publication also stated that some part of the product in an in vitro system produced by a cytoplasmic enzyme must “be vRNA.” However, recently Hastie and Mahy (1973) in nearest neighbor analysis and Specific hybridization confirmed the formation of the cytoplasmic enzyme almost exclusively of cRNA, as was first shown by Scholtissek (1969).Caliguiri and Compans (1973) also isolated RNA polymerase from the cytoplasm of cells infected with influenza virus, which synthesized RNA in an in vitro system without less than 90% of which has a base sequence complementary to hsRNA. Hastie and Mahy (1973) “found that a significant percentage of the product in the in vitro system synthesized by the nuclear enzyme in the presence of actinomycin D was not able to pibridize with unlabeled vRNA. It is not yet clear what RNA type is not capable of such hybridization. Very little of the RNA synthesized under these conditions hybridizes with unlabeled aRNA (Scholtissek, unpublished data).
The kinetics of incorporation of labeled GTP into viral RNA can be interpreted as indicating that there is no reinitiation of RNA synthesis in the in vitro system. If a crude enzyme preparation is incubated at low salt concentrations, almost all of the newly synthesized RNA is initially single-stranded. However, after extraction with phenol, “a large percentage of RNA becomes RNAase-resistant. Phenol converts the intermediate replication structure, consisting of a single-stranded template and newly synthesized cRNA, held together at the replication site by a polymerase molecule, into a partially double-stranded structure (Feix et al., 1967; Oberg, Philipson, 1971). These data on the influenza virus enzyme product in an in vitro system can be interpreted to mean that the polymerase not only initiates and continues polymerization, but it dissociates the newly synthesized chain from its template. Otherwise, a double-stranded RNA structure is formed, which has no biological functions (Paffenholz and Scholtissek, 1973). If incubation is carried out at high salt concentrations or with purified enzyme, a large percentage of the product is already double-stranded RNA before phenol extraction (Schwartz, Scholtissek, 1973 ).
This property of influenza virus RNA polymerase to synthesize exclusively cfRNA in an in vitro system has been exploited.
It is called to establish the genetic relationship of different strains of influenza virus by determining the homology in the base sequence between them (Scholtissek, Rott, 1969b; Hobson, Scholtissek, 1970; Anschutz et al., 1972).
IV. SYNTHESIS OF VIRAL PROTEINS
A. IN VITRO TRANSLATION
The problem of what type of RNA—vir ionic or complementary—is informative for the synthesis of viral proteins has not yet been resolved. Conflicting results have been obtained regarding the type of virus-specific RNA associated with polysomes. Nayak (1970) discovered a sucrose gradient in the polysomal region, mainly vRNA, while Pons (1972) isolated exclusively vcRNA from polysa.The latter was confirmed by the observation that after the addition of actinomycin D, which preferentially affects the synthesis of vcRNA (see section III, B, 1), 2 hours after infection, cfRNA is not detected in polysomes of infected cells (Pons, 1973).
Using a protein synthesis system from E. coli and influenza virus vRNA as a template, Siegert et al. (1973) discovered the formation of viral NP protein in vitro. This labeled NP protein was characterized by gel precipitation using the Ouchterlony test. In contrast, Kingsbury and Webster (1973) did not observe any viral protein synthesis with vRNA using a protein synthesis system derived from rabbit reticulocytes.In the same system, however, they detected the synthesis of viral M protein (om. Chapter 2) on an RNA template isolated from infected cells. Thus, at the moment it is impossible to answer the question whether only viriopic or only complementary, or whether some RNA fragments of one type and some RNA fragments of another type are used as templates for protein synthesis. Therefore, for now, It is difficult for influenza viruses to apply the definition of a “negative” or “positive” viral chain, as proposed by Baltimore (1971).
B. SYNTHESIS OF VIRAL PROTEINS IN VIVO
The study of the synthesis of viral “proteins” is facilitated by the fact that in an infected cell the synthesis of cell polypeptides is replaced by virus-specific synthesis. In chicken embryo fibroblast cells infected with HPV (Joss et al., 1969; Skehel, 1972; Klenk, Rott, 1973), and in BHK 2IF cells infected with the WSN strain of influenza virus (Lasarowitz et al., 1971), to Almost 4 hours after infection are synthesized
only viral proteins (31). Somewhat earlier, researchers observed the synthesis of three or four polypeptides in infected ticks (Taylor et al., 1969; Joss et al., 1969; Holland, Kiehn, 1970; White et al., 1970). Subsequently, other polypeptides were discovered (Lazorowitz et al., 1971; Skehel, 1972; Klenk et al., 1972b; Krug, Etkind, 1973). In general, all structural β-proteins were detected in infected cells: one or two P-proteins, the nucleocapoid subunit NP, membrane protein M, hemagglutinium glycoprotein in uncleaved (HA) and cleaved (HA1 and HA2) forms, and the NA subunit.
In addition to virion proteins, one or two nonstructural proteins (NS) have been described.
There are noticeable differences<в уровнях синтеза отдельных вирусных полипептидов. NP- и NS-полипептиды обычно первыми обнаруживаются в зараженных «летках. Skehel (1973) предположил, что полипептиды Р2, NP и NS, которые первыми обнаруживаются в «клетках, зараженных ВЧП, являются
products of RNA fragments formed during selective transcription by virion polymerase of three fragments of the viral genome. When the cells were infected in the presence of cycloheximide and the pulse tag was added after removal of the antibiotic, only these three polypeptides were detected. Based on this, it was assumed that the RNA molecules for these components were formed with the help of the introduced virion lolimerase during primary transcription. From the 4th to the 6th hour after infection of chicken fibroblasts with HPV, the level of M-protein synthesis increases, and the synthesis of NS-lolileptide decreases (Skehel, 1972, 1973). Thus, levels of iolipeptide synthesis can be individually controlled and may vary during the growth cycle.
Apart from the cleavage of the HA polypeptide into HA1 and HA2, there is no evidence that virus-specific influenza virus polypeptides are obtained as a result of cleavage of large precursors (Taylor et al., 1969; Lazarowitz et al., 1971, Skehel, 1972; Klenk, Rott, 1973 ).
New information has recently been obtained regarding the localization of viral components in infected cells using autoradiography (Becht, 1971) or cell fractionation and gel electrophoresis techniques (Taylor et al., 1969, 1970). According to these studies, the synthesis of all viral proteins appears to occur in the cytoplasm. Previous studies of the localization of nucleotide antigen by immunofluorescence were interpreted as indicating that synthesis occurs in the nucleus with subsequent release of the antigen into the cytoplasm (Liu, 1955; Breitenfeld and Schafer, 1957; Holtermann et al., 1960). However, it is clear that immunofluorescence determines the accumulation of antigen, and not its synthesis (see section IV, B, 2).
1. RNA polymerase
Virus-specific activity of RNA-dependent RNA polymerase can be detected in cells infected with influenza virus between 13 and 3 hours after infection, depending on the cell system used (Scholtissek and Rott, 1969a; Skehel and Burke, 1969; Ruck et al. al., 1969; Mahy, Bromley, 1970). This is the first detectable virus-specific activity after infection. Most of the viral lolimerase activity is detected in the microsomal fraction; some part of this activity remains in the nuclei and cannot be removed from there even by intensive washing. There are no fundamental differences in the kinetics of manifestation or in the necessary cofactors between nuclear and microsomal enzymes (Scholtissek and Rott, 1969a; Mahy et al., 1975).
Upon further fractionation of the cytoplasm in a stepwise sucrose gradient using the method of Caliguiri and Tamm (1970), polymerase activity is detected in rough membranes (Compans and Caliguiri, 1973; Klenk et al., 1974a).
Since viral polymerase activity has been detected in purified virus particles (see Chapter 5), the question arises as to which of the viral proteins it might be associated with. The polymerase isolated from cells infected with influenza virus was purified approximately 200-fold. The only virus-specific product associated with polymerase activity was RNP antigen (NP protein plus viral vRNA, determined by the complement fixation method). All attempts to remove RNA from this complex resulted in a complete loss of enzyme activity (Schwarz and Scholtissek, 1973). The P protein was put forward as a candidate for the role of viral polymerase (Kilbourne et al., 1972). When the enzymatic complex, labeled in vivo with amino acids, was isolated from cells infected with influenza virus and purified approximately 35-fold, electrophoretic analysis initially revealed only HP-beloa in this complex< (Compans, Caliguiri, 1973). Впоследствии, однако, при других условиях введения;метки удалось обнаружить и Р-белок (Caliguiri, Compans, 1974). С другой стороны, Klenk и соавт. (1974) обнаружили Р-белок в цитоплазматическом золе, "который не обладает полимеразной активностью (Scholtissek, Rott, 1969a; Skehel, Burke, 1969). Эти наблюдения могут означать, что Р-белок осуществляет свою (Предполагаемую активность ферментов только при связывании с РНП-антигеном.
It is unlikely that RNP antigen itself has polymerase activity, since hyperimmune serum against RNP antigen does not inhibit polymerase activity, whereas convalescent serum, which may contain antibodies to polymerase, does (Scholtissek et al., 1971) . This convalescent serum (which was obtained from animals infected with influenza A viruses) inhibited the polymerase activity of all influenza A virus strains studied, but did not inhibit the polymerase activity of influenza B virus. All these observations are consistent with the idea that RNP- antigen (BPHK + NP = protein), can serve as a template for the synthesis of cRNA-
2. Nucleocapsid protein
The NP protein binds to the viral RNA, forming the RNP antigen. This is true for the NP protein isolated not only from the virion, but also from the infected cell (Schafer, 1957). “It can be detected for the first time by serological means, when
approximately 3 hours after infection, an hour before the appearance of hemagglutinin (Breitenfeld, Schafer, 1957). After this time, the RNP antigen titer does not increase significantly. This may occur due to an equilibrium between new synthesis and incorporation into mature particles. By labeling, the NP protein can “be detected in infected cells within 2 hours after infection (Scholtissek and Rott, 1961; Krug, 1972).
With the help of fluorescent antibodies, RNP antigen is first detected in the nuclei. Later it appears in the cytoplasm (Breitenfeld and Schafer, 1957). Under certain conditions, such as abortive infection (Franklin, Brietenfeld, 1959), in the presence of p-fluorophenylalanine (Zimmermann, Schafer, 1960), or under conditions of the von Magnus phenomenon (Rott, Scholtissek, 1963), RNP -antigen remains in the nuclei.
The early accumulation of RNP antigen in the nuclei of infected cells does not mean that the NP protein is also synthesized inside the nuclei. Autoradiographic studies, as well as the use of cell fractionation methods, indicate the cytoplasmic synthesis of this and another arginine-rich protein and their rapid transport from the cytoplasm to the nuclei (Taylor et al., 1969, 1970; Becht, 1971).
In extracts of infected cells, a certain fraction of the RNP antigen contains vRNA (Pons, 1971; Krug, 1972; Krug, Etkind, 1973), although only one type of RNA is found in viral particles (which follows from the absence of any self-hybridization of vRNA) (Scholtissek, Rott, 1971; Pons, 1971). It is impossible to decide whether the RNP antigen containing cfRNA has a specific role in the process of viral propagation or whether it is just an artifact that appears during the process of cellular fractionation. It was shown that both RNA strands bind equally well to the NP protein in vitro (Scholtissek and Becht, 1971). Thus, if there is any free NP protein and free cRNA, the corresponding RNP antigen is immediately formed during the homogenization process. Virion RNA can be displaced from the RNP antigen by polyvin "Ilsulfate" M (Pons et al., 1969). Therefore, it can be tested whether replacement of different viral RNAs in RNP antigen is feasible in cellular homogenates. From changes in the base composition of viral RNA labeled for various periods of time with 32P and isolated from the cytoplasmic RNP antigen, Krug (1972) concludes that some of the cfRNA, before it is incorporated into the RNP antigen, exists in a form free from NP- squirrel. The incorporation of 32P into the RNA of animal vesicles occurs with a significant lag phase due to the rather slow incorporation of labeled phosphorus into the x-position of nucleoside triphosphates (Scholtissek, 1965). Until appropriate adjustments are made for calculations of changes in composition
no basis has been made, Krug's (1972) data should be interpreted with caution.
A kinetic analysis of the “appearance of RNP antigen in the nuclei and cytoplasm” carried out by Krug (1972) suggests that the RNP antigen accumulating in the nuclei is not a precursor of the RNP antigen found in the cytoplasm.
3. Non-structural proteins
Several nonstructural virus-specific proteins of unknown function have been described for infected cells. One of them, with a relative molecular weight of 25,000, which accumulates in large quantities, was designated NS (Lazarowitz et al., 1971). In polyacrylamide gels, it has a migration rate close to the mobility of the M protein. However, both proteins, appear to be independent of each other, as can be seen from the differences in their peptide maps. Large amounts of NS protein are found in the nuclei (Lazarowitz et al., 1971; Krug, Etkind, 1973). This information is consistent with the data previous immunofluorescence studies by Dim-mock (1969), who observed “bright staining of nuclei with antiserum specific for non-structural viral antigens, and this staining probably reflected the presence of NS protein. This protein was also found to be the main virus-specific protein in fractions of free and membrane-bound ribosomes isolated from infected cells (Pons, 1972; Compans, 1973; Klenk et al., 1974a). The association of NS with ribosomes appears to depend on the tonal force (Krug and Etkind, 1973). In buffers with low ionic strength, THIS nolipaptide was adsorbed on both ribosomal subunits, whereas upon addition of salt it was removed from them.
A recent study (Gregoriades, 1973) has cast some doubt on the identification of NS as a non-structural polypeptide distinct from the virion polypeptide M. It has been possible to extract the M-polypeptide from the virion with acidic chloroformmethanol, and a protein with identical electrophoresis mobility can also be extracted from whole infected cells, -nuclei or polysomes. Analysis of the products of tryptic processing of the M protein, as well as nuclear and ribosome-associated proteins, led to many matches, suggesting that the M and NS proteins are identical. Necessary, however , further information to provide a convincing explanation for these results.
In addition to NS, there may be other non-structural virus-specific components, although none of them exist. has not been sufficiently characterized. Using antiserum directed against non-structural viral antigens,
Dimmock and Watson (1969) precipitated radiolabeled polypeptides from infected cells. Electrophoretic analysis in polyacrylamide gel suggested the presence of several non-structural polypeptides with a main component corresponding to NS. One of the remaining nonstructural components migrates more rapidly and may correspond to the relative molecular weight component of 10,000 to 15,000 described by Skehel (1972) and Krug and Etkind (1973).
4. Membrane M protein
The M protein, which lines the inner surface of the lipid bilayer of the envelope and is rich in the virion, is found in relatively small quantities in infected cells. This suggests not only the controllability of M protein synthesis, but also the possibility that this synthesis is the rate-limiting stage of virus reproduction (Lazarowitz et al., 1971) This concept is supported by the data that at a temperature of 29 ° C, at which virus production is suppressed, the M protein is the only virus-specific protein that cannot be detected in infected cells (Klenk, Rott , 1973).
M protein (can be found on the smooth and plasma membranes of infected cells (Lazarowitz et al., 1971; Cornpans, 1973a; Klenk et al., 1974a). These data indicate the affinity of this protein for membranes.
5. Hemagglutinin
Hemagglutinin is synthesized as a large glycoprotein - a precursor of HA, which is subsequently cleaved into two smaller glycoproteins: HAi and HA2 "(Lazarowitz et al., 1971). Cleavage, which can be suppressed by protease inhibitors (Klenk, Rott, 1973), is carried out by apparently by proteolytic enzymes of the host cell (Lazarowitz et al., 1973).The degree of cleavage depends on the strain of the virus, the host cell, the level of cytotoxic effect and the presence or absence of serum in the medium (Lazarowitz et al., 1971, 1973a, b ; Klenk, Rott, 1973; Stanley et al., 1973). Thus, WSN grown in a culture of chick embryo fibroblasts contains only cleaved hemagglutnin glish proteins, while such cleavage is virtually absent if WSN virions are grown in MDBK cells in the absence serum. In the presence of serum, however, WSN hemagglutinin is also cleaved. Cleavage in this
system takes place on the plasma membrane (Lazaro-witz et al., 1973a). In the VChP system, the mechanism of such splitting is apparently different. Cleavage “occurs at intracellular membranes, and plasminogen in this case is not necessary (Shchepk et al., 1974a). The degree of splitting decreases sharply at 25 °C (Klenk and Rott, 1973).
HA cleavage is not a requirement for hemagglutinating activity or virion assembly (La-zarowitz et al., 1973a; Stanley et al., 1973), but recent studies have found that it is required for infectivity (Klenk et al. , 1975b). These data are consistent with the hypothesis that, in addition to its role in adsorption, HA* has another function in the infection process and that degradation is necessary for this function. On the basis that degradation of HA is a phenomenon dependent on " host cell, and that particles containing uncleaved HA are low infectivity, suggest that the host range and spread of influenza virus infection is dependent on the presence of a host cell protease as an activating enzyme.
In experiments on fractionation of “letlets infected with vi
Rus influenza, it was found that HA glycoproteins are always
are associated with membranes (Compans, 1973a; Klenk et al.,.
1974a). Intracellular localization of these proteins and their moment
walkie-talkie from rough membranes to smooth endoplasmic membranes
sky reticulum and to the plasma membranes will be de
are described in detail in section VII, B. .,
6. Neuraminidase
Viral NA* as an active enzyme was discovered
3 hours after infection in the chorionic allantoic membrane
nakh, and by extrapolation it is established that the beginning of its syn
thesis occurs 1-2 hours after infection (Noll et al.,
1961). The intracellular localization of NA* was studied using
then cell fractionation, and found that she apparently
mu, is similar to the localization of HA* (Compans, 1973a; Klenk et al.,
1974a). NA* was found in association with membranes,
obtained from the smooth endoplasmic reticulum,
when determining it by biological activity and by analysis
zu in polyazhrilamide gel. Enzyme activity was high
dena also in fractions containing rough membranes
us. These data are consistent with data obtained from
immunofluorescence (Maeno, Kilbourne, 1970). After 4 hours
after infection, neuraminidase can be detected in the cytoplasm
plasma; later she appears to concentrate on peri
cell feria.
V. SYNTHESIS OF CARBOHYDRATES
Carbohydrates are involved in the formation of glycoproteins and glycolylides of the influenza virus envelope (Klenk et al., 1972a). Glycolipids of myxoviruses (origin from the plasma membranes of the host cell (Klenk, Choppin, 1970), but it has not been determined which is predominantly included in the virion: pre-existing or newly synthesized glycolipids.
The use of radioactive precursors such as glucosamine, mannose, galactose and fucose, which are specifically incorporated into viral glucopeltides, has shown that the carbohydrate side chains of these glycopeptides are resynthesized during infection (Haslam et al., 1970; Cornpans et al., 1970a; Schwarz and Klenk, 1974). Cell fractionation experiments have provided additional information about the sites of glycosylation of viral glycoproteins. Glucosamine is associated with HA polypeptide in both smooth and rough cytoplasmic membrane fractions; however, fucose is associated with HA in smooth but not rough membranes (Compans, 1973b). .Suppression of protein synthesis with puromycin almost immediately stops the incorporation of glucosamine, while fucose continues to be incorporated for about 10-15 minutes (Stanley et al., 1973).Finally, in FPV, the glycoprotein is the precursor of HA and cleavage products of HAj<и НА2 содержат, по-видимому, полный состав маннозы « глюкозамина, тогда как содержание фукозы и галактозы значительно выше в продуктах расщепления (Klenk et al., 1975a; Schwarz, Klenk, 1974). Эта наблюдения предполагают, что биосинтез углеводных боковых целей гликопротеинов НА осуществляется по стадиям с различными остатками Сахаров, добавляемыми в разных участках клетки. Глюкозамин и манноза (присоединяются, по-видимому, к полипептидам НА на шероховатых мембранах вскоре после или даже в процессе синтеза поляпептида, в то время как фукоза, вероятно, прикрепляется позже с помощью трансфераз, присутствующих в гладких мембранах.
These glycosyltransferases are probably cellular enzymes. Therefore, the carbohydrate (part of the glycoproteins is apparently determined by the host cell. There is, however, evidence that in addition to these host cell enzymes, viral NA* plays a significant role in the formation of carbohydrate side chains. It has been established that the surface of the shells of myceoviruses lacks neuraminic acid (Klenk, Choppin, 1970b; Klenk et al., 1970), while in viral shells that do not contain this enzyme, such carbohydrate is a common component (Klenk, Choppin, 1971; McSharry, Wagner, 1971 ; Renkonen et al., 1971) These data suggest that the
The effect of neuraminic acid is an essential feature of myxoviruses. It was recently shown that NA* is responsible for removing neuraminic acid from the influenza virus envelope, thereby preventing the formation of receptors on the viral envelope that would otherwise lead to the formation of large aggregates of viral particles (Palese et al., 1974). These data support the concept that the carbohydrate part of NA*, as the main surface glycoprotein, is a product of the combined action of (Cellular transeferases and viral NA*. Thanks to its action, the virus is able to introduce a virus-specific modification into (Initially, a host-specific, complex structure of carbohydrate modification, which, according to -apparently essential for the biological activity of the virus.
D-glucosamine and 2-deoxy-O-glucose inhibit the formation of biologically active HA*, NA* and infectious virus (Kilbourne, 1959; Kaluza et al., 1972). Biochemical studies have revealed that these sugars compete with the biosynthesis of viral glycoproteins (Ghandi et al., 1972; Klenk et al., 1972b). In the presence of these inhibitors, the size of glycoprotein NA decreases. The degree of reduction depends on the dose. Thus, with an increase in the concentration of sugar, glycoprotein NA with a relative molecular weight of 76,000 gradually turns into a compound with a molecular weight of 64,000, which was designated as HA0 (Klenk et al., 1972b; Schwarz and Klenk, 1974).The shift in molecular weight parallels the decrease in carbohydrate content and the HA0 protein was found to be almost free of carbohydrates (Schwarz and Klenk, 1974).These results indicate that HA0 is an incompletely glycosylated or "eglycosylated polypeptide chain of the HA glycoprotein and that the inhibitory effect of D-glucose amine a and 2-deoxy-O-glucose is due to damage to glycosylation. The HA0 polypeptide is associated with membranes, like normal HA It also migrates from the rough to the smooth reticulum, where it is cleaved into polypeptides HA01 and HA02. Therefore, carbohydrates are probably not essential for the affinity of this polypeptide for the membrane. However, the absence of hemagglutinating activity in infected cells suggests that the non-glycosylated protein is unable to bind to receptors.
VI. LIPID SYNTHESIS
Like all enveloped viruses, influenza virus acquires its lipids by recycling host cell lipids. This position is confirmed by the following observations:
Denia. The lipid composition of the influenza virus was found to be similar to that of the host cell (Ambruster and Beiss, 1958; Frommhagen et al., 1959). Host cell lipids, radioactively labeled before infection, are incorporated into viral particles (Wecker, 1957). When the virus is grown in various host cells, modifications of viral lipids are detected (Kates et al., 1961, 1962). In general, the lipids of viruses (which bud from the cell surface, closely reflect the lipid composition of the plasma membrane of the host cell (Klenk, Choppin, 1969; 1970a, b; Renko-nen et al., 1971). The level of de novo synthesis f- ospholylides in chick embryo fibroblast cells remained unchanged for 7 hours after influenza virus infection, after which all lipid synthesis was suppressed (Blough et al., 1973).This suppression is probably not a primary effect, but may be secondary to in relation to inhibition of RNA or protein synthesis or to “other biological effects.
Thus, based on the results obtained to date, it can be assumed that the synthesis of viral lipids occurs through normal cellular lipid biosynthetic processes, and the viral envelope is formed by the incorporation of lipids from the plasma membrane of the host cell.
VII. ASSEMBLY (see also Chapter 2)
A. FORMATION OF NUCLEOCAPSIDS
As already mentioned, it is most likely that the nucleocapsid protein is synthesized in the cytoplasm. Apparently, it is present there for a short time in free form, and then associates with viral RNA, forming nucleocapsids (Klenk et al., 1974; Compans and Caliguiri, 1973). Since the NP protein is quickly incorporated into yaucleocapsids, RNA can be selected from the reformed pool (Krug, 1972). Due to the small size of influenza virus nucleocapsids, they cannot be accurately identified in infected cells using electron microscopy. Clusters of filaments or fibers with a diameter of about 5 nm observed in the cytoplasm possibly represent viral ribonucleoproteins (Apostolov et al., 1970; Compans et al., 1970b).
Available data indicate that the RNA genome of influenza virus virions consists of 5-7 fragments (see Chapter 6).
Therefore, any infectious particle needs at least one copy of each fragment. Hirst (1962) pre-
posited that nucleocapsids from the intracellular pool can be included in (virions randomly. The proportion of infectious virions in the E population can be increased by including additional RNA fragments in the average virion (Compans et al., 1970). For example, if five are required for infectious different RNA fragments, each virnoy contains a total of 7 fragments included in the virnoy randomly, then approximately 22% of the virions should be infectious.Evidence for the random inclusion of RNA fragments was strengthened by the recent observations of Hirst (1973) that in a viral population, recombination occurs between particles that do not form plaques.The ability of such particles to participate in recombination can be explained by the absence of one or more fragments in the particles, with the missing fragments varying from one particle to another, so that suitable particles of a defective virus can form recombinants.
B. PROCESS OF VIRUS BUNDING
Like most enveloped viruses, the influenza virus assembles on preformed cell membranes; assembly occurs by budding from the plasma membrane. The first demonstration of virus release from the cell by a process not involving lysis was provided by Murphy and Bang (1952) in early electron microscopy studies of cells infected with influenza virus. Filamentous and ■ spherical structures. Virus particles were not visible inside the cells during the formation of infectious virus and it was therefore clear that the virus particles were forming on the cell surface. Using ferritin-labeled antibodies, Morgan et al. (1961) observed that the cell surface contained viral antigen in the areas where the virus is formed. Later electron microscopic studies showed that the surface of the budding virus contains the same membrane as the host cell with a layer of projections corresponding to the viral “spikes” on the outer surface. On the inner On the surface of the viral membrane there is an additional electron-dense layer absent on the cell surface, which probably consists of M-olipeptides (Bachi et al., 1969; Compans, Dimmock, 1969; Apostolov et al., 1970).
Electron microscope studies have given grounds to ■suggest the order in which the viral components of the as-
associate on the cell membrane (Bachi et al., 1961; Compans and Dimmock, 1969; Compans et al., 1970b).
Viral envelope proteins appear first, being included in some areas of the membrane that should have normal morphology; however, the observed specific adsorption of erythrocytes on these areas of the membrane indicates the presence of HA protein here. Then, apparently, the M protein associates with the inner surface of such areas of the membrane, forming an electron-dense layer. Next, the ribonucleoprotein specifically binds to the membrane in these areas and the process of budding occurs by bending and protruding the membrane segment and surrounding the associated ribonucleoprotein. Polyacrylamide gel electrophoresis data also support the idea that envelope proteins associate with the plasma membrane more quickly than RNPs (Lazarowitz et al., 1971).Host cell polypeptides are displaced from the membrane, which is a precursor to the viral envelope, since such polypeptides do not found in purified virions.As already mentioned, neuraminic acid residues are absent from the envelope of budding influenza virus particles, but are present in adjacent areas of the cell membrane (Klenk et al., 1970).
These data provide evidence of a dramatic transition in chemical composition between the envelope of the budding viral particle and the adjacent cell membrane.
However, on the other hand, an important feature of the budding process is that the viral envelope is continuous with the plasma membrane of the host cell and is morphologically similar to it (Compans and Dimmock, 1969). As mentioned, the lipids in these membranes have a very close resemblance to the lipids of the host cell membrane. These observations suggest that lipids in the intact plasma membrane readily exchange with lipids in the budding virus particles by radial diffusion.
Consequently, the envelope of the budding virion is formed from a small region of the cell membrane modified by the inclusion of virion envelope proteins. This concept, of course, does not imply the need for the synthesis of all components of the membrane at the plasma membrane.
Indeed, it has been known for a long time that the constituents of the membrane must migrate considerable distances from one part of the cell to another in order to get from the site of their biosynthesis to the site of membrane assembly. Breitenield and Schafer (1957) showed that in cells infected with influenza virus, HA* can first be seen
occur in all parts of the cell and that it is localized in the perinuclear zone in increased concentration. Later, HA* accumulates in the "peripheral region" of the flare and can also be demonstrated in thin threads that protrude from the flare membrane.
The idea that envelope components migrate from inside the cell to the surface has recently been confirmed and expanded by a series of studies using cell fractionation and analysis of viral proteins in different cell fractions. These studies also suggest that the HA glycoprotein, and possibly other envelope proteins, are synthesized on the rough endoplasmic reticulum (Compans, 1973a; Klenk et al., 1974). As revealed in pulse-chase experiments, after a few minutes HA is found in the membranes of the smooth endoplasmic reticulum (Compans, 1973a; Stanley et al., 1973; Klenk et al., 1974) and in the plasma membrane (Stanley et al., 1974). ., 1973). Although chase experiments from the smooth endoplasmic reticulum to the plasma membrane have not been performed, it seems plausible that HA migrates from the rough endoplasmic reticulum to the plasma membrane, bypassing the smooth endoplasmic reticulum. It should be noted that during the entire time of such migration, HA and other membrane proteins are integral parts of the membranes along which they move; OBI is never found as dissolved proteins.
In the smooth membrane fractions, which are believed to be derived primarily from the endoplasmic reticulum, all major envelope proteins are found (Compans 1973a; Klenk et al., 1974). However, their relative quantities here differ from those in the plasma membrane and virion (Stanley et al., 1973; Klenk et al., 1974). The ratio of M protein to glycoprotein HA is higher in the envelope of the mature virion than in the membranes of the endoplasmic reticulum. These data suggest that only a small amount of membranes carrying the HA glycoprotein are converted into the viral envelope, namely the membrane fractions that contain carbohydrate-free proteins. As already indicated, the synthesis of the M protein may be the stage that limits the process of virus assembly.
The difference in the rate of synthesis of different envelope proteins supports the hypothesis that the assembly of the envelope is a multistage process. This concept is consistent with the question of the process of formation of HA*, including the sequential addition of the carbohydrate moiety and proteolytic cleavage during the migration of the primary gene product.
VIII. RELEASE OF THE FLU VIRUS
The problem of releasing the influenza virus from the host cell,
appears to be closely related to the problem of viral function
NA*, which has already been discussed in detail earlier (see
cases V and ch. 4). The fact that this enzyme plays an essential role
role in the release of the virus, derived from the ability of anti
bodies specific to NA* suppress such release (Se-
to, Rott, 1966; Webster et al. G968). Moreover, such antibodies
prevent virus elution from erythrocytes (Brown, Laver,
1968). Bacterial NA*, which is not inhibited by antibodies
lami to viral NA*, is capable of releasing the virus from cells,
treated with such antibodies (Compans et al., 1969;
Webster, 1970). On the other hand, divalent antibodies
to NA also cause virus aggregation (Seto and Chang, 1969;
Compans et al., 1969; Webster, 1970), and monovalent anti
bodies do not prevent the release of the virus, although they inhibit
more than 90% of neuraminidase activity (Becht et al., 1971).
All these data taken together suggest that
divalent antibodies interfere with the release of the virus
by binding it to antigens present on the
cell surface, and by inhibiting the activity of enzymes
cop. The role of neuraminidase in the release of the display virus
or Palese et al. (G974), who established that this fer
ment is necessary for the removal of neuroamylic acid from vi
Russian surface to avoid aggregation of virions - later -
kov on the cell surface.
IX. ABNORMAL FORMS OF REPRODUCE
A. ABORTIVE REPRODUCTION DEPENDING ON THE HOST CELL
Influenza viruses can infect a wide variety of host cells. However, in many of the infected cells the yield of infectious progeny is either very low or not detectable, although viral components can be detected in normal titers. This type of host cell-dependent interruption of the infectious cycle is called abortive infection. This abortive infectious cycle was originally observed in mice , infected into the brain with non-neurotropic strains of the influenza virus (Schlesinger, 1953). The higher the administered dose of the virus, the greater the amount of newly synthesized hemagglutinin. Several other host cell-influenza A virus systems have been described in which only RNP-^ were produced antigen and hemagglutinin, but not an infectious virus. In all these ENT systems studied so far, the RNP antigen accumulated in the nucleus and was not detected
armed with the help of fluorescent antibodies in the cytoplasm (Henle et al., 1955; Franklin, Breitenfeld, 1959; Ter Meulen, Love, 1967; Fraser, 1967).
B. VON MAGNUS PHENOMENON
During serial passages of influenza viruses at a multiplicity higher than 1 (Barry, 1961), increasing amounts of incomplete virus are formed, emerging from the host cell (von Magnus, 1951, 1952). These viral particles have a surface structure very similar on the structure of the infectious virus, are immunogenic: and cause homologous interference.They contain less RNA and RNP antigen, exhibit a lower ratio of infectivity to hemagglutinating activity and contain more lipids than complete virus particles (von Magnus, 1954; Isaacs, 1959; Pauker et al., 1959; Rott and Schafer, 1961; Rott and Scholtissek, 1963).
When analyzing the RNA of a partial virus, it was found that viral RNA with a relatively high molecular weight is either absent or present in reduced quantities, while the amount of RNA with a low molecular weight increases (Duesberg, 1968; Pons, Hirst, 1969; Nayak, 1969) .
In cells infected with the second undiluted passage of the influenza virus, all the genetic information of the virus is present, since cfRNA isolated from these cells is capable of converting, after hybridization, labeled RNA isolated from the infectious virus into a completely RNA azoresistant form (Scholtissek, Rott , 1969b). Thus, the increase in the amount of low molecular weight RNA in incomplete particles may be due to high molecular weight RNA, which can be included in these particles in the form of destroyed and, therefore, non-functioning molecules. This idea is supported by data that with a thirsty undiluted passage, the ability to produce infectious virus first decreases, after which the synthesis of hemagglutinin, neuraminidase and, finally, RNP antigen decreases (Scholtissek et al., 1966).
Nayak (1972) found that during the first high-multiplicity passage, the virus emerging early was fully infectious and produced a normal pattern of infectious virus RNA fragments in the sucrose gradient, whereas the virus emerging later had an RNA profile typical of an incomplete (background) -Magnus) virus1.
- Dihydrotestosterone in men - function, norm and pathology
- Getting insulin: all the main ways
- How steroids affect the prostate gland How to protect the prostate during the course
- Not a virus, not a protein, but its name is prion. Prions for short
- Proper nutrition for stomach ulcers
- Therapeutic back massage for scoliosis Differentiated back massage 10 15
- How to remove phlegm from the bronchi at home
- Cancer neck plant. Snake mountaineer. Cancer neck root
- What are the benefits of kombucha?
- What is dropsy: types and treatment
- Flax seeds for kidney treatment
- How does the influenza virus work?
- Excess protein: how harmful is it?
- Testosterone standards during doping control
- How to correctly use atropine for complex treatment Atropine farm action
- What is hypogonadism in men and how to treat this disease
- The wrist joint is the perfect motor apparatus of the upper limb
- Phosphatidylcholine - fat burning injections
- When is dihydrotestosterone elevated in women and men?
- Diet for thyrotoxicosis of the thyroid gland What foods are not allowed for thyrotoxicosis