It is called the absorbed dose. Radiation doses and units of measurement. The concept of collective dose
(Russian designation: Gr; international: Gy). The previously used non-system unit rad is equal to 0.01 Gy.
Does not reflect the biological effect of radiation (see equivalent dose).
Encyclopedic YouTube
1 / 2
More about radiation
More about Radiation
Subtitles
Hello. In this episode of the TranslatorsCafe.com channel we will talk about ionizing radiation or radiation. We will look at sources of radiation, ways to measure it, and the effect of radiation on living organisms. We will talk in more detail about such radiation parameters as absorbed dose rate, as well as equivalent and effective doses of ionizing radiation. Radiation has many uses, from generating electricity to treating cancer patients. In this video, we will discuss how radiation affects the tissues and cells of humans, animals, and biomaterials, with a particular focus on how quickly and how severely damage occurs to irradiated cells and tissues. Radiation is a natural phenomenon that manifests itself in the fact that electromagnetic waves or elementary particles with high kinetic energy move within a medium. In this case, the medium can be either matter or vacuum. Radiation is all around us, and our life without it is unthinkable, since the survival of humans and other animals without radiation is impossible. Without radiation on Earth there will be no such natural phenomena as light and heat necessary for life. There would be no cell phones or Internet. In this video we will discuss a special type of radiation, ionizing radiation or radiation, which is all around us. Ionizing radiation has energy sufficient to remove electrons from atoms and molecules, that is, to ionize the irradiated substance. Ionizing radiation in the environment can arise due to either natural or artificial processes. Natural sources of radiation include solar and cosmic radiation, certain minerals such as granite, and radiation from certain radioactive materials such as uranium and even ordinary bananas, which contain the radioactive isotope potassium. Radioactive raw materials are mined in the depths of the earth and used in medicine and industry. Sometimes radioactive materials enter the environment as a result of industrial accidents and in industries that use radioactive raw materials. Most often this occurs due to non-compliance with safety rules for storing and working with radioactive materials or due to the absence of such rules. It is worth noting that until recently, radioactive materials were not considered hazardous to health. On the contrary, they were used as healing drugs, and they were also valued for their beautiful glow. Uranium glass is an example of a radioactive material used for decorative purposes. This glass glows fluorescent green due to the addition of uranium oxide. The percentage of uranium in this glass is relatively small and the amount of radiation it emits is small, so uranium glass is considered relatively safe for health. They even made glasses, plates and other utensils from it. Uranium glass is prized for its unusual glow. The sun emits ultraviolet light, so uranium glass glows in sunlight, although this glow is much more pronounced under ultraviolet light lamps. In radiation, higher energy photons (ultraviolet) are absorbed and lower energy photons (green) are emitted. As you have seen, these beads can be used to test dosimeters. You can buy a bag of beads on eBay.com for a couple of dollars. First let's look at some definitions. There are many ways to measure radiation, depending on what exactly we want to know. For example, one can measure the total amount of radiation in a given location; you can find the amount of radiation that disrupts the functioning of biological tissues and cells; or the amount of radiation absorbed by a body or organism, and so on. Here we will look at two ways to measure radiation. The total amount of radiation in the environment, measured per unit time, is called the total dose rate of ionizing radiation. The amount of radiation absorbed by the body per unit time is called the absorbed dose rate. The absorbed dose rate is found using information about the total dose rate and the parameters of the object, organism, or part of the body that is exposed to radiation. These parameters include mass, density and volume. Absorbed and exposure dose values are similar for materials and tissues that absorb radiation well. However, not all materials are like this, so often the absorbed and exposure doses of radiation differ, since the ability of an object or body to absorb radiation depends on the material from which it is composed. For example, a sheet of lead absorbs gamma radiation much better than an aluminum sheet of the same thickness. We know that a large dose of radiation, called the acute dose, causes health risks, and the higher the dose, the greater the health risk. We also know that radiation affects different cells in the body differently. Cells that undergo frequent division, as well as unspecialized cells, are most affected by radiation. For example, cells in the embryo, blood cells, and cells of the reproductive system are most susceptible to the negative effects of radiation. At the same time, skin, bones, and muscle tissue are less susceptible to radiation. But radiation has the least effect on nerve cells. Therefore, in some cases, the overall destructive effect of radiation on cells that are less exposed to radiation is less, even if they are exposed to more radiation, than on cells that are more exposed to radiation. According to the theory of radiation hormesis, small doses of radiation, on the contrary, stimulate the body's defense mechanisms, and as a result the body becomes stronger and less susceptible to disease. It should be noted that these studies are at an early stage, and it is not yet known whether such results will be obtained outside the laboratory. Now these experiments are carried out on animals and it is unknown whether these processes occur in the human body. Due to ethical considerations, it is difficult to obtain permission for such research involving human participants. Absorbed dose is the ratio of the energy of ionizing radiation absorbed in a given volume of a substance to the mass of the substance in this volume. Absorbed dose is the main dosimetric quantity and is measured in joules per kilogram. This unit is called gray. Previously, the non-systemic unit rad was used. The absorbed dose depends not only on the radiation itself, but also on the material that absorbs it: the absorbed dose of soft X-rays in bone tissue can be four times the absorbed dose in air. At the same time, in a vacuum the absorbed dose is zero. The equivalent dose, which characterizes the biological effect of irradiation of the human body with ionizing radiation, is measured in sieverts. To understand the difference between dose and dose rate, we can draw an analogy with a kettle into which water is poured from the tap. The volume of water in the kettle is the dose, and the filling speed, depending on the thickness of the water stream, is the dose rate, that is, the increment in the radiation dose per unit time. Equivalent dose rate is measured in sieverts per unit of time, for example microsieverts per hour or millisieverts per year. Radiation is generally invisible to the naked eye, so special measuring instruments are used to determine the presence of radiation. One widely used device is a dosimeter based on a Geiger-Muller counter. The counter consists of a tube in which the number of radioactive particles is counted, and a display that displays the number of these particles in different units, most often as the amount of radiation over a certain period of time, for example per hour. Instruments with Geiger counters often produce short beeps, such as clicks, each of which indicates that a new emitted particle or particles have been counted. This sound can usually be turned off. Some dosimeters allow you to select the click frequency. For example, you can set the dosimeter to make a sound only after every twentieth particle counted or less often. In addition to Geiger counters, dosimeters also use other sensors, such as scintillation counters, which make it possible to better determine what type of radiation currently predominates in the environment. Scintillation counters are good at detecting both alpha, beta and gamma radiation. These counters convert the energy released during radiation into light, which is then converted in a photomultiplier into an electrical signal, which is measured. During measurements, these counters work over a larger surface area than Geiger counters, so they measure more efficiently. Ionizing radiation has very high energy and therefore ionizes the atoms and molecules of biological material. As a result, electrons are separated from them, which leads to a change in their structure. These changes are caused by ionization weakening or breaking the chemical bonds between particles. This damages molecules inside cells and tissues and disrupts their function. In some cases, ionization promotes the formation of new bonds. The disruption of cell function depends on how much radiation damages their structure. In some cases, disorders do not affect cell function. Sometimes the work of cells is disrupted, but the damage is minor and the body gradually restores the cells to working condition. Such disturbances often occur during the normal functioning of cells, and the cells themselves return to normal. Therefore, if the level of radiation is low and the damage is minor, then it is quite possible to restore the cells to their normal state. If the radiation level is high, then irreversible changes occur in the cells. With irreversible changes, cells either do not work as they should or stop working altogether and die. Damage by radiation to vital and essential cells and molecules, such as DNA and RNA molecules, proteins or enzymes, causes radiation sickness. Damage to cells can also cause mutations, which can cause the children of patients whose cells are affected to develop genetic diseases. The mutations can also cause cells in patients to divide too quickly - which in turn increases the likelihood of cancer. Today, our knowledge about the effects of radiation on the body and the conditions under which this effect is aggravated is limited, since researchers have very little material at their disposal. Much of our knowledge is based on research into the medical records of victims of the atomic bombings of Hiroshima and Nagasaki, as well as victims of the Chernobyl nuclear power plant explosion. It is also worth noting that some studies of the effects of radiation on the body, which were carried out in the 50s - 70s. last century, were unethical and even inhumane. In particular, these are studies conducted by the military in the United States and the Soviet Union. Most of these experiments were conducted at test sites and designated areas for testing nuclear weapons, such as the Nevada Test Site in the United States, the Soviet Nuclear Test Site on Novaya Zemlya, and the Semipalatinsk Test Site in what is now Kazakhstan. In some cases, experiments were carried out during military exercises, such as during the Totsk military exercises (USSR, in what is now Russia) and during the Desert Rock military exercises in Nevada, USA. During these exercises, researchers, if you can call them that, studied the effects of radiation on the human body after atomic explosions. From 1946 to the 1960s, experiments on the effects of radiation on the body were also carried out in some American hospitals without the knowledge or consent of the patients. Thank you for your attention! If you liked this video, please don't forget to subscribe to our channel!
It is known that radioactive radiation under certain conditions can pose a danger to the health of living organisms. What is the reason for the negative effects of radiation on living beings?
The fact is that α-, β- and γ-particles, passing through a substance, ionize it, knocking electrons out of molecules and atoms. Ionization of living tissue disrupts the vital activity of the cells that make up this tissue, which negatively affects the health of the entire organism.
The more energy a person receives from the flow of particles acting on him and the smaller the person’s mass (i.e., the more energy falls on each unit of mass), the more serious disturbances in his body this will lead to.
- The energy of ionizing radiation absorbed by the irradiated substance (in particular, body tissues) and calculated per unit mass is called the absorbed dose of radiation
The absorbed radiation dose D is equal to the ratio of the energy E absorbed by the body to its mass m:
The SI unit of absorbed dose of radiation is the gray (Gy).
From this formula it follows that
1 Gy = 1 J / 1 kg
This means that the absorbed radiation dose will be equal to 1 Gy if 1 J of radiation energy is transferred to a substance weighing 1 kg.
In certain cases (for example, when soft tissues of living beings are irradiated with X-ray or γ-radiation), the absorbed dose can be measured in roentgens (R): 1 Gy corresponds to approximately 100 R.
The greater the absorbed dose of radiation, the more harm (other things being equal) this radiation can cause to the body.
But for a reliable assessment of the severity of the consequences that can result from the action of ionizing radiation, it is also necessary to take into account that with the same absorbed dose, different types of radiation cause biological effects of different magnitudes.
Biological effects caused by any ionizing radiation are usually assessed in comparison with the effect of X-rays or γ-radiation. For example, at the same absorbed dose, the biological effect from α-radiation will be 20 times greater than from γ-radiation, from the action of fast neutrons the effect can be 10 times greater than from γ-radiation, from the action of β- radiation - the same as from γ-radiation.
In this regard, it is customary to say that the quality factor of α-radiation is 20, the above-mentioned fast neutrons are 10, while the quality factor of γ-radiation (as well as X-ray and β-radiation) is considered equal to unity. Thus,
- quality factor K shows how many times the radiation hazard from exposure to a living organism of a given type of radiation is greater than from exposure to γ-radiation (at the same absorbed doses)
To assess biological effects, a quantity called equivalent dose.
The equivalent dose H is determined as the product of the absorbed dose D and the quality factor K:
The equivalent dose can be measured in the same units as the absorbed dose, but there are also special units for its measurement.
The SI unit of equivalent dose is the sievert (Sv). Submultiple units are also used: millisievert (mSv), microsievert (μSv), etc.
From this formula it follows that for X-ray, γ- and β-radiation (for which K = 1) 1 Sv corresponds to an absorbed dose of 1 Gy, and for all other types of radiation - a dose of 1 Gy multiplied by the quality factor corresponding to this radiation .
When assessing the effects of ionizing radiation on a living organism, it is also taken into account that some parts of the body (organs, tissues) are more sensitive than others. For example, at the same equivalent dose, cancer is more likely to occur in the lungs than in the thyroid gland. In other words, each organ and tissue has a certain radiation risk coefficient (for the lungs, for example, it is 0.12, and for the thyroid gland - 0.03).
The absorbed and equivalent doses also depend on the time of irradiation (i.e., on the time of interaction of radiation with the environment). All other things being equal, these doses are greater the longer the irradiation time, i.e. the doses accumulate over time.
When assessing the degree of danger that radioactive isotopes pose to living beings, it is also important to take into account the fact that the number of radioactive (i.e., not yet decayed) atoms in a substance decreases over time. In this case, the number of radioactive decays per unit time and the emitted energy decrease proportionally.
Energy, as you already know, is one of the factors that determines the degree of negative effects of radiation on a person. Therefore, it is so important to find a quantitative relationship (i.e., a formula) by which one could calculate how many radioactive atoms remain in a substance at any given point in time.
To derive this dependence, you need to know that the rate of decrease in the number of radioactive nuclei varies for different substances and depends on a physical quantity called half-life.
- Half-life T is the period of time during which the original number of radioactive nuclei is halved on average
Let us derive the dependence of the number N of radioactive atoms on time t and the half-life T. We will count time from the moment the observation began t 0 = 0, when the number of radioactive atoms in the radiation source was equal to N 0 . Then after a period of time
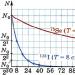
The formula is called the law of radioactive decay. It can be written in another form, for example. From the last formula it follows that the greater T, the less 2 t/T and the greater N (for given values of N 0 and t). This means that the longer the half-life of an element, the longer it “lives” and emits, posing a danger to living organisms. This is also confirmed by the graphs of N versus t presented in Figure 165, constructed for the isotopes of iodine (T I = 8 days) and selenium (T Se = 120 days).

Rice. 165. Graph of the number of radioactive atoms versus time for isotopes of iodine and selenium
You should know how to protect yourself from radiation. Under no circumstances should radioactive drugs be handled; they must be handled with special tongs with long handles.
It is easiest to protect yourself from α-radiation, since it has low penetrating ability and is therefore retained, for example, by a sheet of paper, clothing, or human skin. At the same time, α-particles that enter the body (with food, air, through open wounds) pose a great danger.
β-Radiation has a much greater penetrating power, making it more difficult to protect against. β-Radiation can travel up to 5 m in air; it is capable of penetrating into body tissues (approximately 1-2 cm). Protection against β-radiation can be, for example, a layer of aluminum several millimeters thick.
γ-radiation has even greater penetrating power; it is retained by a thick layer of lead or concrete. Therefore, γ-radioactive drugs are stored in thick-walled lead containers. For the same reason, nuclear reactors use a thick concrete layer that protects people from γ-rays and various particles (α-particles, neutrons, nuclear fission fragments, etc.).
Questions
- What is the reason for the negative effects of radiation on living beings?
- What is the absorbed dose of radiation? Does radiation cause more harm to the body at a higher or lower dose, if all other conditions are the same?
- Do different types of ionizing radiation cause the same or different biological effects in a living organism? Give examples.
- What does the radiation quality factor show? What quantity is called the equivalent radiation dose?
- What other factor (besides energy, type of radiation and body mass) should be taken into account when assessing the effects of ionizing radiation on a living organism?
- What percentage of atoms of a radioactive substance will remain after 6 days if its half-life is 2 days?
- Tell us about ways to protect yourself from exposure to radioactive particles and radiation.
1.What is the reason for the negative impact of radiation on living beings?
Ionizing radiation passing through living tissue knocks out electrons from molecules and atoms, destroying it, which negatively affects human health.
2. What is the absorbed dose of radiation? Does radiation cause more harm to the body at a higher or lower dose, if all other conditions are the same?
3. Do different types of ionizing radiation cause the same or different biological effects in a living organism? Give examples.
Different types of ionizing radiation have different biological effects. For A-radiation it is 20 times greater than for ϒ-radiation.
4. What does the radiation quality factor show? What quantity is called the equivalent radiation dose?

5. What other factor (besides energy, type of radiation and body weight) should be taken into account when assessing the effects of ionizing radiation on a living organism?
When assessing the impact of ionizing radiation on a living organism, one should also take into account the time of its exposure, since radiation doses accumulate, as well as the different sensitivity of body parts to this radiation, which is taken into account using the radiation risk coefficient.
6. What percentage of atoms of a radioactive substance will remain after 6 days if its half-life is 2 days?

7. Tell us about methods of protection from exposure to radioactive particles and radiation.
To protect against radioactivity, you should avoid contact with such substances, never pick them up, and be careful not to get them inside. In all cases, radioactive radiation, depending on its nature, has different penetrating abilities; for some types of radiation it is enough to avoid direct contact (radiation); protection from others can be provided by distance or thin layers of an absorber (house walls, metal car body) or thick layers concrete or lead (hard γ-radiation).
Length and distance converter Mass converter Converter of volume measures of bulk products and food products Area converter Converter of volume and units of measurement in culinary recipes Temperature converter Converter of pressure, mechanical stress, Young's modulus Converter of energy and work Converter of power Converter of force Converter of time Linear speed converter Flat angle Converter thermal efficiency and fuel efficiency Converter of numbers in various number systems Converter of units of measurement of quantity of information Currency rates Women's clothing and shoe sizes Men's clothing and shoe sizes Angular velocity and rotation frequency converter Acceleration converter Angular acceleration converter Density converter Specific volume converter Moment of inertia converter Moment of force converter Torque converter Specific heat of combustion converter (by mass) Energy density and specific heat of combustion converter (by volume) Temperature difference converter Coefficient of thermal expansion converter Thermal resistance converter Thermal conductivity converter Specific heat capacity converter Energy exposure and thermal radiation power converter Heat flux density converter Heat transfer coefficient converter Volume flow rate converter Mass flow rate converter Molar flow rate converter Mass flow density converter Molar concentration converter Mass concentration in solution converter Dynamic (absolute) viscosity converter Kinematic viscosity converter Surface tension converter Vapor permeability converter Water vapor flow density converter Sound level converter Microphone sensitivity converter Converter Sound Pressure Level (SPL) Sound Pressure Level Converter with Selectable Reference Pressure Luminance Converter Luminous Intensity Converter Illuminance Converter Computer Graphics Resolution Converter Frequency and Wavelength Converter Diopter Power and Focal Length Diopter Power and Lens Magnification (×) Converter electric charge Linear charge density converter Surface charge density converter Volume charge density converter Electric current converter Linear current density converter Surface current density converter Electric field strength converter Electrostatic potential and voltage converter Electrical resistance converter Electrical resistivity converter Electrical conductivity converter Electrical conductivity converter Electrical capacitance Inductance Converter American Wire Gauge Converter Levels in dBm (dBm or dBm), dBV (dBV), watts, etc. units Magnetomotive force converter Magnetic field strength converter Magnetic flux converter Magnetic induction converter Radiation. Ionizing radiation absorbed dose rate converter Radioactivity. Radioactive decay converter Radiation. Exposure dose converter Radiation. Absorbed dose converter Decimal prefix converter Data transfer Typography and image processing unit converter Timber volume unit converter Calculation of molar mass Periodic table of chemical elements by D. I. Mendeleev
Initial value
Converted value
rad millirad joule per kilogram joule per gram joule per centigram joule per milligram gray exagray petagray theragray gigagray megagray kilogray hectogray decagray decigray centigray milligray microgray nanogray picogray femtogray attogray sievert millisievert microsievert nausea and vomiting weakness headache fatigue fever infection diarrhea i leukopenia purpura bleeding hair loss cover dizziness and disorientation hypertension electrolyte imbalance mortality
Read more about absorbed dose of radiation
General information

Radiation can be ionizing or non-ionizing. This article will talk about the first type of radiation, its use by people, and the harm it brings to health. Absorbed dose differs from exposure dose in that it measures the total amount of energy absorbed by an organism or substance, rather than a measure of air ionization resulting from the presence of ionizing radiation in the environment.
Absorbed and exposed dose values are similar for materials and tissues that absorb radiation well, but not all materials are like that, so absorbed and exposed radiation doses are often different because the ability of an object or body to absorb radiation depends on the material it is made of. For example, a sheet of lead absorbs gamma radiation much better than an aluminum sheet of the same thickness.

Units for measuring absorbed radiation dose
One of the most widely used units of measurement of absorbed radiation dose is gray. One gray (Gy) is the dose of radiation when one kilogram of matter absorbs one joule of energy. This is a very large amount of radiation, much more than a person usually receives during exposure. From 10 to 20 Gy is a lethal dose for an adult. Therefore, tenths (decigrays, 0.1 Gy), hundredths (centigrays, 0.01 Gy), and thousandths (milligrays, 0.001 Gy) of grays are often used, along with smaller units. One Gy is 100 rad, that is, one rad is equal to a centigray. Despite the fact that rad is an outdated unit, it is often used today.
The amount of radiation that a body absorbs does not always determine the amount of harm caused to the body by ionizing radiation. To determine harm to the body, dose equivalent units are often used.

Equivalent radiation dose
Units for measuring absorbed radiation dose are often used in the scientific literature, but most non-specialists are not very familiar with them. In the media, units of equivalent radiation dose are more often used. With their help, it is easy to explain how radiation affects the body as a whole and tissues in particular. Radiation equivalent dose units help provide a more complete picture of the harms of radiation because they are calculated by taking into account the degree of damage caused by each type of ionizing radiation.
The damage caused to tissues and organs of the body by different types of ionizing radiation is calculated using the quantity relative biological effectiveness of ionizing radiation. If two identical bodies are exposed to radiation of the same type with the same intensity, then the relative effectiveness and equivalent dose are equal. If the types of radiation are different, then these two quantities are different. For example, the harm caused by beta, gamma or X-rays is 20 times weaker than the harm caused by irradiation with alpha particles. It is worth noting that alpha rays cause harm to the body only if the radiation source enters the body. Outside the body, they are practically harmless, since the energy of alpha rays is not enough even to penetrate the top layer of skin.
The equivalent radiation dose is calculated by multiplying the absorbed radiation dose by the coefficient of biological effectiveness of radioactive particles for each type of radiation. In the example above, this coefficient for beta, gamma and x-rays is one, and for alpha rays it is twenty. An example of equivalent radiation dose units is banana equivalent and sieverts.
Sieverts
Sieverts measure the amount of energy absorbed by a body or tissue of a certain mass during radiation exposure. Sieverts are also commonly used to describe the harm that radiation causes to people and animals. For example, the lethal dose of radiation for humans is 4 sieverts. A person with such a dose of radiation can sometimes be saved, but only if treatment is started immediately. At 8 sieverts, death is inevitable, even with treatment. People usually receive much smaller doses, so millisieverts and microsieverts are often used. 1 millisievert is equal to 0.001 sievert, and 1 microsievert is 0.000001 sievert.
Banana equivalent

Banana equivalent measures the dose of radiation a person receives when eating one banana. This dose can also be expressed in sieverts - one banana equivalent is equal to 0.1 microsieverts. Bananas are used because they contain a radioactive isotope of potassium, potassium-40. This isotope is also found in some other foods. Some examples of banana equivalent measurements: An X-ray at the dentist is equivalent to 500 bananas; a mammogram - 4000 bananas, and a lethal dose of radiation - 80 million bananas.
Not everyone agrees with using the banana equivalent, since radiation from different isotopes affects the body differently, so comparing the effect of potassium-40 with other isotopes is not entirely correct. Also, the amount of potassium-40 is regulated by the body, so when the amount in the body increases, such as after a person has eaten a few bananas, the body excretes the excess potassium-40 to keep the balance of the amount of potassium-40 in the body constant.
Effective dose
The units described above are used to determine the amount of radiation that affected not the body as a whole, but a specific organ. When different organs are irradiated, the risk of cancer is different, even if the absorbed dose of radiation is the same. Therefore, in order to find out the harm caused to the body as a whole, if only a certain organ is irradiated, an effective dose of radiation is used.
The effective dose is found by multiplying the absorbed radiation dose by the radiation severity factor for that organ or tissue. The researchers who developed the system for calculating the effective dose used information not only about the likelihood of cancer from radiation, but also about how a patient's life would be shortened and worsened by radiation and the cancer that comes with it.
Like the equivalent dose, the effective dose is also measured in sieverts. It is important to remember that when we talk about radiation measured in sieverts, we can be talking about either an effective dose or an equivalent dose. Sometimes this is clear from the context, but not always. If sieverts are mentioned in the media, especially in the context of accidents, disasters, and accidents related to radiation, then most often they mean an equivalent dose. Very often, those who write about such problems in the media do not have enough information about which parts of the body are affected or will be affected by radiation, so it is impossible to calculate the equivalent dose.

The effect of radiation on the body
Sometimes it is possible to estimate the damage caused to the body by radiation by knowing the absorbed radiation dose in grays. For example, the radiation a patient is exposed to during local radiation therapy is measured in grays. In this case, it is also possible to determine how such localized radiation will affect the body as a whole. The total amount of radiation absorbed during radiotherapy is usually high. When this value exceeds 30 Gy, damage to the salivary and sweat glands, as well as other glands, is possible, which causes dry mouth and other unpleasant side effects. Total doses exceeding 45 Gy destroy hair follicles, leading to irreversible hair loss.
It is important to remember that even when the total dose of radiation absorbed is quite high, the degree of damage to tissues and internal organs depends on the total amount of time the radiation is absorbed, that is, on the intensity of absorption. So, for example, a dose of 1,000 rad or 10 Gy is lethal if received within a few hours, but it may not even cause radiation sickness if received over a longer period of time.
Unit Converter articles were edited and illustrated by Anatoly Zolotkov
Do you find it difficult to translate units of measurement from one language to another? Colleagues are ready to help you. Post a question in TCTerms and within a few minutes you will receive an answer.
The main characteristic of the interaction of ionizing radiation and the environment is the ionization effect. In the initial period of development of radiation dosimetry, it was most often necessary to deal with X-ray radiation propagating in the air. Therefore, the degree of ionization of the air in X-ray tubes or devices was used as a quantitative measure of the radiation field. A quantitative measure based on the amount of ionization of dry air at normal atmospheric pressure, fairly easy to measure, is called exposure dose.
Exposure dose determines the ionizing ability of X-rays and gamma rays and expresses the radiation energy converted into the kinetic energy of charged particles per unit mass of atmospheric air. Exposure dose is the ratio of the total charge of all ions of the same sign in an elementary volume of air to the mass of air in this volume.
The SI unit of exposure dose is the coulomb divided by kilogram (C/kg). Non-systemic unit - x-ray (R). 1 C/kg = 3880 R
Absorbed dose
When expanding the range of known types of ionizing radiation and the areas of its application, it turned out that the measure of the impact of ionizing radiation on matter cannot be easily determined due to the complexity and diversity of the processes occurring in this case. An important one, which gives rise to physicochemical changes in the irradiated substance and leads to a certain radiation effect, is the absorption of the energy of ionizing radiation by the substance. As a result of this, the concept arose absorbed dose. The absorbed dose shows how much radiation energy is absorbed per unit mass of any irradiated substance and is determined by the ratio of the absorbed energy of ionizing radiation to the mass of the substance.
In SI units, absorbed dose is measured in joules divided by kilogram (J/kg), and has a special name - Gray (Gr). 1 Gy- this is the dose at which the mass 1 kg energy of ionizing radiation is transferred 1 J. The extrasystemic unit of absorbed dose is glad.1 Gy=100 rad.
The absorbed dose is a fundamental dosimetric quantity; it does not reflect the biological effect of radiation.
Equivalent dose
Equivalent dose (E,HT,R) reflects the biological effect of radiation. The study of individual consequences of irradiation of living tissues has shown that, with the same absorbed doses, different types of radiation produce different biological effects on the body. This is due to the fact that a heavier particle (for example, a proton) produces more ions per unit path in the tissue than a lighter particle (for example, an electron). For the same absorbed dose, the higher the radiobiological destructive effect, the denser the ionization created by the radiation. To take this effect into account, the concept was introduced equivalent dose. The equivalent dose is calculated by multiplying the value of the absorbed dose by a special coefficient - the coefficient of relative biological effectiveness ( OBE) or the quality factor of a given type of radiation ( WR), reflecting its ability to damage body tissue.
When exposed to different types of radiation with different quality factors, the equivalent dose is determined as the sum of equivalent doses for these types of radiation.
The SI unit of equivalent dose is sievert (Sv) and is measured in joules divided by kilogram ( J/kg). Magnitude 1 Sv equal to the equivalent dose of any type of radiation absorbed in 1 kg biological tissue and creating the same biological effect as the absorbed dose in 1 Gy photon radiation. The non-systemic unit of measurement of equivalent dose is Bare(before 1963 - biological equivalent x-ray, after 1963 - biological equivalent glad). 1 Sv = 100 rem.
Quality factor - in radiobiology, the average coefficient of relative biological effectiveness (RBE). Characterizes the danger of this type of radiation (compared to γ-radiation). The higher the coefficient, the more dangerous this radiation is. (The term should be understood as “harm quality coefficient”).
The values of the quality factor of ionizing radiation are determined taking into account the impact of the microdistribution of absorbed energy on the adverse biological consequences of chronic human exposure to low doses of ionizing radiation. For the quality factor there is GOST 8.496-83. GOST as a standard is used to control the degree of radiation hazard for persons exposed to ionizing radiation during work. The standard is not used for acute exposures and during radiotherapy.
RBE of a particular type of radiation is the ratio of the absorbed dose of X-ray (or gamma) radiation to the absorbed dose of radiation at the same equivalent dose.
| Quality factors for types of radiation: | |
| Photons (γ-rays and X-rays), by definition | 1 |
| β-radiation (electrons, positrons) | 1 |
| Muons | 1 |
| α-radiation with energy less than 10 MeV | 20 |
| Neutrons (thermal, slow, resonant), up to 10 keV | 5 |
| Neutrons from 10 keV to 100 keV | 10 |
| Neutrons from 100 keV to 2 MeV | 20 |
| Neutrons from 2 MeV to 20 MeV | 10 |
| Neutrons over 2 MeV | 5 |
| Protons, 2…5 MeV | 5 |
| Protons, 5…10 MeV | 10 |
| Heavy recoil cores | 20 |
Effective dose
Effective dose, (E, effective equivalent dose) - a quantity used in radiation protection as a measure of the risk of long-term effects of radiation ( stochastic effects) the entire human body and its individual organs and tissues, taking into account their radiosensitivity.
Different parts of the body (organs, tissues) have different sensitivity to radiation exposure: for example, with the same radiation dose, cancer is more likely to occur in the lungs than in the thyroid gland. The effective equivalent dose is calculated as the sum of equivalent doses for all organs and tissues, multiplied by the weighting factors for these organs, and reflects the total effect of radiation on the body.
Weighted coefficients are established empirically and calculated in such a way that their sum for the entire organism is unity. Units effective dose match the units of measurement equivalent dose. It is also measured in Sievertach or Barach.
Fixed effective equivalent dose (CEDE - the committed effective dose equivalent) is an estimate of radiation doses per person resulting from inhalation or consumption of a certain amount of radioactive substance. CEDE is expressed in rem or sieverts (Sv) and takes into account the radiosensitivity of various organs and the time during which the substance remains in the body (up to a lifetime). Depending on the situation, CEDE may also refer to radiation dose to a specific organ rather than the entire body.
Effective and equivalent dose- these are standardized values, i.e. values that are a measure of damage (harm) from the effects of ionizing radiation on a person and his descendants. Unfortunately, they cannot be directly measured. Therefore, operational dosimetric values have been introduced into practice, unambiguously determined through the physical characteristics of the radiation field at a point, as close as possible to the standardized ones. The main operating quantity is ambient dose equivalent(synonyms - ambient dose equivalent, ambient dose).
Ambient dose equivalent H*(d)— dose equivalent that was created in the spherical phantom ICRE(International Commission on Radiation Units) at a depth d (mm) from the surface along a diameter parallel to the direction of radiation, in a radiation field identical to that considered in composition, fluence and energy distribution, but monodirectional and uniform, i.e. Ambient dose equivalent H*(d) is the dose that a person would receive if he were present at the location where the measurement is being taken. Ambient dose equivalent unit - sievert (Sv).
Group doses
By calculating the individual effective doses received by individuals, one can arrive at a collective dose - the sum of individual effective doses in a given group of people over a given period of time. The collective dose can be calculated for the population of an individual village, city, administrative-territorial unit, state, etc. It is obtained by multiplying the average effective dose by the total number of people who were exposed to radiation. The unit of measurement for collective dose is man-sievert (people-sv.), non-systemic unit - person-rem (person-rem).
In addition, the following doses are distinguished:
- commitment- expected dose, half-century dose. Used in radiation protection and hygiene when calculating absorbed, equivalent and effective doses from incorporated radionuclides; has the dimension of the corresponding dose.
- collective- a calculated value introduced to characterize the effects or damage to health from exposure of a group of people; unit - sievert (Sv). The collective dose is defined as the sum of the products of average doses and the number of people in dose intervals. The collective dose can accumulate over a long period of time, not even one generation, but covering subsequent generations.
- threshold- dose below which manifestations of this radiation effect are not observed.
- maximum permissible doses (MAD)- the highest values of the individual equivalent dose for a calendar year, at which uniform exposure over 50 years cannot cause adverse changes in health that can be detected by modern methods (NRB-99)
- preventable- predicted dose due to a radiation accident that can be prevented by protective measures.
- doubling- a dose that increases by 2 times (or by 100%) the level of spontaneous mutations. The doubling dose is inversely proportional to the relative mutational risk. According to currently available data, the doubling dose for acute exposure is on average 2 Sv, and for chronic exposure it is about 4 Sv.
- biological dose of gamma neutron radiation- a dose of gamma radiation equally effective in damaging the body, taken as standard. Equal to the physical dose of a given radiation multiplied by the quality factor.
- minimally lethal- the minimum radiation dose that causes the death of all irradiated objects.
Dose rate
Dose rate (irradiation intensity) is the increment of the corresponding dose under the influence of a given radiation per unit of time. It has the dimension of the corresponding dose (absorbed, exposure, etc.) divided by a unit of time. Various special units may be used (for example, microR/hour, Sv/hour, rem/min, cSv/year and etc.).
- Features of the structure of the internal organs of fish
- Trapezius muscle (upper portion) Trapezius muscle - location, functions, causes of pain
- How to protect yourself from ticks How to protect yourself from ticks in the city
- IUD for pregnancy: when to install, principle of action, types
- What is a synchrophasotron
- Serum creatinine (with GFR determination)
- Nutrition for hepatitis C - healthy and prohibited foods
- Diet after gallbladder removal - what can and cannot be eaten?
- What does an encephalogram of the brain show?
- Amg hormone, what is it?
- What is Botkin's disease and what are its symptoms?
- Radiation doses and units of measurement
- What substances are formed during respiration?
- Symptoms and help for poisoning with psychotropic drugs How to recover from an overdose
- Organs of the circulatory system: structure and functions
- Production and use of sulfur
- Non-communicable diseases
- Electrical conductivity
- Wild plants: names and photos
- Human and social health