Carbonates. Baking soda formula. Baking soda: formula, application Safety of baking soda for humans
Sodium carbonate Na 2 CO 3 . soda ash. White, melts and decomposes when heated. Sensitive to moisture and carbon dioxide in the air. Forms decahydrate ( crystalline soda). It is highly soluble in water, hydrolyzes at the anion, and creates a highly alkaline environment in solution. Decomposes with strong acids. Restored with coke. Enters into ion exchange reactions.
Qualitative reaction to the CO 3 2‑ ion – the formation of a white precipitate of barium carbonate, decomposed by strong acids (HCl, HNO 3) with the release of carbon dioxide.
It is used for the synthesis of sodium compounds, eliminating the “permanent” hardness of fresh water, in the production of glass, soap and other detergents, cellulose, mineral paints, enamels. In nature it is found in ground brines and brine of salt lakes.
Equations of the most important reactions:

Receipt V industry (Solvay's method 1861–1863):
a) a mixture of NH 3 and CO 2 is passed through a saturated NaCl solution:
NaCl + NH 3 + H 2 O + CO 2 = NH 4 Cl + NaHCO 3 ↓
(under these conditions, baking soda is slightly soluble);
b) the NaHCO 3 precipitate is subjected to dehydration ( calcination):
2NaHCO 3 = Na 2 CO 3+ H 2 O + CO 2
Potassium carbonate K 2 CO 3. Oxosol. Technical name potash. White, hygroscopic. Melts without decomposition, and decomposes upon further heating. Sensitive to moisture and carbon dioxide in the air. Very soluble in water, hydrolyzes at the anion, creating a highly alkaline environment in solution. Decomposes with strong acids. Enters into ion exchange reactions.
It is used in the production of optical glass, liquid soap, mineral paints, many potassium compounds, as a dehydrating agent.
Equations of the most important reactions:

Receipt V industry :
a) heating potassium sulfate [natural raw materials - minerals Cainite KMg(SO 4)Cl ZH 2 O and schoenite K 2 Mg(SO 4) 2 6H 2 O] with slaked lime Ca(OH) 2 in a CO atmosphere (pressure = 15 atm):
K 2 SO 4 + Ca(OH) 2 + 2СО = 2K(HCOO) + CaSO 4
b) calcination of potassium formate K(HCOO) in air:
2K(HCOO) + O 2 = K 2 CO 3 + H 2 O + CO 2
Sodium bicarbonate NaHCO 3. Acid oxo salt. Technical name baking soda. White friable powder. When heated slightly, it decomposes without melting; when wet, it begins to decompose at room temperature. Moderately soluble in water, hydrolyzes the anion to a small extent. Decomposes by acids, neutralized by alkalis. Enters into ion exchange reactions.
Qualitative reaction on the HCOd ion - the formation of a white precipitate of barium carbonate under the action of barite water and the decomposition of the precipitate by strong acids (HCl, HNO 3) with the release of carbon dioxide. It is used in the food industry as a medicine.
Equations of the most important reactions:

Receipt: saturation of a solution of Na 2 CO 3 (see) with carbon dioxide.
Calcium carbonate CaCO 3. Oxosol. A common natural substance, the main component of sedimentary rock - limestone (its varieties - chalk, marble, calcareous tuff, marl), pure CaCO 3 in nature is a mineral calcite. White, decomposes when heated, melts under excess pressure of CO 2. Insoluble in water (= 0.0007 g/100 g H 2 O).
Reacts with acids, ammonium salts in hot solution, coke. It is transferred into solution by the action of excess carbon dioxide with the formation of bicarbonate Ca(HCO 3) 2 (exists only in solution), which determines the “temporary” hardness of fresh water (together with magnesium and iron salts). Hardness removal (water softening) is carried out by boiling or neutralization with slaked lime.
Used for the production of CaO, CO 2, cement, glass and mineral fertilizers [including lime nitrate Ca(NO 3) 2 4H 2 O], as a filler for paper and rubber, building stone (crushed stone) and a component of concrete and slate, in the form of precipitated powder - for the production of school crayons, tooth powders and pastes, mixtures for whitewashing premises.
Equations of the most important reactions:

Baking soda, or drinking soda, is a compound widely known in medicine, cooking, and household consumption. This is an acidic salt, the molecule of which is formed by positively charged sodium and hydrogen ions, and the anion of the acidic residue of carbonic acid. The chemical name of soda is sodium bicarbonate or sodium bicarbonate. Formula of the compound according to the Hill system: CHNaO 3 (gross formula).
The difference between sour salt and medium salt
Carbonic acid forms two groups of salts - carbonates (medium) and bicarbonates (acidic). The trivial name for carbonates - soda - appeared in ancient times. It is necessary to distinguish between medium and acid salts by names, formulas and properties.
Na 2 CO 3 - sodium carbonate, disodium carbonate, washing soda ash. Serves as a raw material for the production of glass, paper, soap, and is used as a detergent.
NaHCO 3 - sodium bicarbonate. The composition suggests that the substance is a monosodium salt of carbonic acid. This compound is distinguished by the presence of two different positive ions - Na + and H +. Externally, the crystalline white substances are similar, they are difficult to distinguish from each other.
The substance NaHCO 3 is considered baking soda not because it is used internally to quench thirst. Although this substance can be used to prepare a fizzy drink. A solution of this bicarbonate is taken orally in case of increased acidity of gastric juice. In this case, the excess H + protons are neutralized, which irritate the walls of the stomach, causing pain and burning.
Physical properties of baking soda
Bicarbonate is white monoclinic crystals. This compound contains atoms of sodium (Na), hydrogen (H), carbon (C) and oxygen. The density of the substance is 2.16 g/cm3. Melting point - 50-60 °C. Sodium bicarbonate is a milky white powder, a solid, finely crystalline compound, soluble in water. Baking soda does not burn, and when heated above 70 ° C, it decomposes into sodium carbonate, carbon dioxide and water. In production conditions, granulated bicarbonate is more often used.

Safety of baking soda for humans
The compound is odorless and its taste is bitter and salty. However, it is not recommended to smell or taste the substance. Inhaling sodium bicarbonate may cause sneezing and coughing. One use is based on baking soda's ability to neutralize odors. The powder can be used to treat sports shoes to get rid of unpleasant odors.
Baking soda (sodium bicarbonate) is a harmless substance in contact with the skin, but in solid form it can cause irritation to the mucous membrane of the eyes and esophagus. In low concentrations, the solution is non-toxic and can be taken orally.
Sodium bicarbonate: compound formula
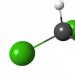
The gross formula CHNaO 3 is rarely found in equations of chemical reactions. The fact is that it does not reflect the connection between the particles that form sodium bicarbonate. The formula commonly used to characterize the physical and chemical properties of a substance is NaHCO 3 . The relative arrangement of atoms is reflected by the ball-and-stick model of the molecule:

If you find out from the periodic table the atomic masses of sodium, oxygen, carbon and hydrogen. then you can calculate the molar mass of the substance sodium bicarbonate (formula NaHCO 3):
Ar(Na) - 23;
Ar(O) - 16;
Ar(C) - 12;
Ar(H) - 1;
M (CHNaO 3) = 84 g/mol.
Structure of matter
Sodium bicarbonate is an ionic compound. The crystal lattice includes the sodium cation Na +, which replaces one hydrogen atom in carbonic acid. The composition and charge of the anion is HCO 3 -. Upon dissolution, partial dissociation occurs into ions that form sodium bicarbonate. The formula reflecting the structural features looks like this:

Solubility of baking soda in water
7.8 g of sodium bicarbonate dissolves in 100 g of water. The substance undergoes hydrolysis:
NaHCO 3 = Na + + HCO 3 - ;
H 2 O ↔ H + + OH - ;
When summing up the equations, it turns out that hydroxide ions accumulate in the solution (weakly alkaline reaction). The liquid turns phenolphthalein pink. The color of universal indicators in the form of paper strips in a soda solution changes from yellow-orange to gray or blue.
Exchange reaction with other salts
An aqueous solution of sodium bicarbonate enters into ion exchange reactions with other salts, provided that one of the newly formed substances is insoluble; or a gas is formed, which is removed from the reaction sphere. When interacting with calcium chloride, as shown in the diagram below, both a white precipitate of calcium carbonate and carbon dioxide are obtained. Sodium and chlorine ions remain in the solution. Molecular equation of the reaction:

Interaction of baking soda with acids
Sodium bicarbonate reacts with acids. The ion exchange reaction is accompanied by the formation of salt and weak carbonic acid. At the moment of receipt, it decomposes into water and carbon dioxide (evaporates).
The walls of the human stomach produce hydrochloric acid, which exists in the form of ions
H + and Cl - . If you take sodium bicarbonate orally, reactions occur in a solution of gastric juice with the participation of ions:
NaHCO 3 = Na + + HCO 3 - ;
HCl = H + + Cl - ;
H 2 O ↔ H+ + OH -;
HCO 3 - + H + = H 2 O + CO 2.
Doctors do not recommend constantly using sodium bicarbonate in case of increased stomach acidity. The instructions for the drugs list various side effects of daily and long-term use of baking soda:
- increased blood pressure;
- belching, nausea and vomiting;
- anxiety, poor sleep;
- decreased appetite;
- stomach ache.

Getting Baking Soda
In the laboratory, sodium bicarbonate can be obtained from soda ash. The same method was used previously in chemical production. The modern industrial method is based on the interaction of ammonia with carbon dioxide and the poor solubility of baking soda in cold water. Ammonia and carbon dioxide (carbon dioxide) are passed through the sodium chloride solution. Ammonium chloride and sodium bicarbonate solution are formed. When cooled, the solubility of baking soda decreases, then the substance is easily separated by filtration.
Where is sodium bicarbonate used? Use of baking soda in medicine
Many people know that sodium metal atoms vigorously interact with water, even its vapor in the air. The reaction begins actively and is accompanied by the release of a large amount of heat (combustion). Unlike atoms, sodium ions are stable particles that do not harm a living organism. On the contrary, they take an active part in regulating its functions.
How is a substance, sodium bicarbonate, which is non-toxic to humans and useful in many respects, used? The application is based on the physical and chemical properties of baking soda. The most important areas are household consumption, food industry, healthcare, traditional medicine, and beverages.
Among the main properties of sodium bicarbonate is the neutralization of increased acidity of gastric juice, short-term elimination of pain due to hyperacidity of gastric juice, gastric ulcer and duodenal ulcer. The antiseptic effect of baking soda solution is used in the treatment of sore throat, cough, intoxication, and seasickness. Wash the oral and nasal cavities and mucous membranes of the eyes with it.

Various dosage forms of sodium bicarbonate are widely used, such as powders, which are dissolved and used for infusion. Solutions are prescribed for patients to take orally, and burns are washed with acids. Sodium bicarbonate is also used to make tablets and rectal suppositories. The instructions for the drugs contain a detailed description of the pharmacological action and indications. The list of contraindications is very short - individual intolerance to the substance.
Using baking soda at home
Sodium bicarbonate is an “ambulance” for heartburn and poisoning. Using baking soda at home, you can whiten your teeth, reduce inflammation during acne, and wipe the skin to remove excess oily secretions. Sodium bicarbonate softens water and helps clean dirt from various surfaces.
When hand washing wool knitwear, you can add baking soda to the water. This substance refreshes the color of fabric and removes the smell of sweat. Often, when ironing silk products, yellow marks from the iron appear. In this case, a paste of baking soda and water will help. The substances must be mixed as quickly as possible and applied to the stain. When the paste dries, it should be cleaned with a brush and the product should be rinsed in cold water.
In the reaction with acetic acid, sodium acetate is obtained and carbon dioxide is rapidly released, foaming the entire mass: NaHCO 3 + CH 3 COOH = Na + + CH 3 COO - + H 2 O + CO 2. This process occurs whenever, in the manufacture of fizzy drinks and confectionery, baking soda is “quenched” with vinegar.

The taste of baked goods will be more delicate if you use lemon juice rather than store-bought synthetic vinegar. As a last resort, you can replace it with a mixture of 1/2 tsp. citric acid powder and 1 tbsp. l. water. Baking soda with acid is added to the dough as one of the last ingredients so that you can immediately put the baked goods in the oven. In addition to sodium bicarbonate, ammonium bicarbonate is sometimes used as a leavening agent.
Air, filtered liquid and wash water from the inside of drum 7 go to separator 11, where the air is separated from the liquid phase and goes to the PVFL.
The filtrate from the separator 11 through the barometric pipe 12 goes to the filter liquid collector 13, from where it is pumped out by pump 14 for distillation.
When the drum rotates, the layer of sodium bicarbonate adhering to the filter surface falls under the squeezing roller 6 to eliminate the cracks that form on the surface of the sediment, through which air and wash water can enter the drum. After the squeezing roller, the sediment is washed with a weak liquid or water coming from the pressure tank 4 for rinsing water into the trough 3, which distributes the water in an even stream across the width of the drum. The amount of water supplied for washing is regulated using a tap installed between the pressure tank 4 and trough 3. The washing water is mixed with the filter liquid inside the drum and goes with it to the separator 11.
The washed sodium bicarbonate is again compacted by the second squeezing roller 6 in the direction of rotation of the drum, dried by air sucked through the sediment layer and supplied through pipeline 5, and cut from the filter fabric by knife 8 to conveyor 10, which supplies raw sodium bicarbonate to the soda oven.
Calcination of sodium bicarbonate
Calcination - the thermal decomposition of sodium bicarbonate - is the final stage in the production of soda ash. The main purpose of the calcination department is to obtain a certain amount of soda ash in the form of a continuous material flow.
Technical sodium bicarbonate should be white. The appearance of color indicates corrosion of steel apparatus in the absorption and carbonization sections. The sediment is colored by iron oxide that enters it as a result of corrosion.
The calcification process can be shown by the equation:
2 NaHCO3(solid)=Na2CO3(solid)+CO2(gas)+H2O(steam).
In addition to this main reaction, when heating technical bicarbonate, additional reactions can occur:
(NH4)2CO3↔2NH3(gas)+СО2(gas)+Н2О(steam),
NH4 HCO3↔2NH3(gas)+СО2(gas)+Н2О(steam).
Ammonium chloride reacts when heated with sodium bicarbonate according to the reaction
NH4Cl(solv.)+ NaHCO3 (solv)↔NaCl(solv)+ NH3(gas)+СО2(gas)+Н2О.
Sodium carbamate in the presence of water when heated transforms into soda according to the reaction
2NaCO2NH2+ Н2О↔ Na2CO3(solid)+СО2(gas)+2NH3(gas).
Thus, as a result of calcination, Na2CO3 and NaCl remain in the solid phase, and NH3, CO2 and H2O pass into the gas phase.
The presence of moisture in bicarbonate complicates the design of the apparatus, since wet sodium bicarbonate is not free-flowing, clumps and sticks to the walls of the apparatus. The latter is explained by the fact that moisture, which is a saturated solution of NaHCO3, intensively evaporates upon contact with a hot surface. The released solid phase, crystallizing, forms a crust that tightly adheres to the surface.
A solid layer of soda, which has low thermal conductivity, impairs heat transfer, and in soda stoves heated from the outside by flue gases, it leads to overheating and burnout of the furnace wall. To combat this phenomenon, wet sodium bicarbonate is mixed with hot soda (retur). In this case, a new solid phase is formed - trona (NaHCO3 Na2CO3 2 H2O). Free moisture is bound into crystallization moisture, and the product becomes free-flowing.
During the calcination of sodium bicarbonate and trona, CO2, NH3 and water vapor are released into the gas phase. Ammonia and carbon dioxide must be returned to production. Carbon dioxide is used in the carbonization process of ammoniated brine, for which it is useful to have a gas with a high CO2 content.
The crystallization process can be divided into three periods in time. The first period is characterized by a rapid rise in temperature. Decomposition of bicarbonate is observed, and all the heat is spent on heating the material, removing water of crystallization from the throne and decomposing ammonium carbonate salts. The second period is characterized by a constant temperature of the material (t~125°C). The supplied heat is spent on the thermal decomposition of NaHCO3. In the third period, the temperature of the reaction mass begins to increase sharply. This indicates that the bicarbonate decomposition process has ended and the supplied heat is spent on heating the resulting soda. In practice, to speed up the decomposition process of NaHCO3, the soda temperature at the furnace outlet is kept within 140 – 160°C.
Technological diagram of the calcination process
Rice. 11. Scheme of calcination separation:
1- steam condenser; 2-feed mixer; 3.15 – cell feeders; 4.10 – belt conveyors; 5 – vibrating feeder; 6 – chute-hopper; 7-plow dumper; 8,9,14,16-transporters; 11-cyclone; 12-calcination gas collector; 13-separator; 17-condensate collector; 18-centrifugal pumps; 19-collector of weak liquid; 20-calcination gas cooler; 21-reducing cooling unit (ROU); 22 - calcination gas washer; 23 - washing liquid collector.
Wet sodium bicarbonate washed in filters from a common belt conveyor 10 with a plow dumper 7 is fed into the hopper 6 of a vibrating feeder 5, from where the vibrating feeder and belt conveyor 4 through a cell feeder 3 are fed into the mixer 2. The mixer receives return soda and soda separated from the calcination gases in cyclone 11.
The trona prepared in the mixer is directed into the inter-tubular space of the calciner drum 1. As a result of heat treatment, the trona produces soda ash and calcination gases. Soda ash is removed from the calciner through a cell feeder 15 and enters the conveyor system 8, 9, 16. Soda is taken from the inclined conveyor 8 through the feeder into the mixer. The rest of the soda is transported to the warehouse by conveyors 9 and 14.
Calcination gases are removed from the calciner through mixer 2, in which a vacuum is created using a compressor. On the way to the compressor, the gases undergo dry cleaning in cyclones 11 and wet cleaning in the shop calcination gas manifold 12 and washer 22. Before the washer, the calcination gases are cooled in refrigerator 20.
For irrigation, a so-called weak liquid is supplied to the calcination gas collector, which is formed by the condensation of water vapor in the calcination gas refrigerator. This liquid, in contact with the gas, partially absorbs ammonia and soda dust, then flows into collection 19.
In refrigerator 20, gas passes from top to bottom through the inter-tube space, and cooling water moves in countercurrent in the tubes. To prevent crystallization of the refrigerator tubes and to better flush the gas from soda dust, the inter-tube space is irrigated with a weak liquid. In the washer, the gas is irrigated with water, while it is additionally cooled and completely washed of soda and ammonia.
To heat the calciner, high-pressure water steam is supplied. Before being fed into the calciner, it passes through a reduction cooling unit (RCU), where its temperature is reduced to 270°C and the pressure to 3 MPa. Steam condenses in the calciner tubes, giving off heat to the calcined material. The condensate from the calciner is discharged into the condensate collector 17 and then into the expanders, where it is converted into low-pressure steam.
Soda
(natron, sodium bicarbonate, sodium bicarbonate) - sodium salt that neutralizes acid. Baking soda is sodium bicarbonate NaHCO 3, sodium bicarbonate. In general, "soda" is the technical name for sodium carbonic acid salts H 2 CO 3. Depending on the chemical composition of the compound, baking soda (baking soda, sodium bicarbonate, sodium bicarbonate, sodium bicarbonate) - NaHCO 3, soda ash (sodium carbonate, anhydrous sodium carbonate) - Na 2 CO 3 and crystalline soda - Na 2 CO 3 are distinguished. 10H 2 O, Na 2 CO 3 .7H 2 O, Na 2 CO 3 .H 2 O. Artificial baking soda (NaHCO3) is a white crystalline powder.
Modern soda lakes are known in Transbaikalia and Western Siberia; Lake Natron in Tanzania and Lake Searles in California are very famous. Trona, which is of industrial importance, was discovered in 1938 as part of the Eocene Green River sequence (Wyoming, USA).
In the USA, natural soda satisfies more than 40% of the country's need for this mineral. In Russia, due to the lack of large deposits, soda is not extracted from minerals.
Soda was known to man approximately one and a half to two thousand years BC, and perhaps even earlier. It was mined from soda lakes and extracted from a few deposits in the form of minerals. The first information about the production of soda by evaporating water from soda lakes dates back to 64 AD. Until the 18th century, alchemists in all countries imagined it as a certain substance that hissed with the release of some kind of gas under the action of acids known by that time - acetic and sulfuric. During the time of the Roman physician Dioscorides Pedanius, no one had any idea about the composition of soda. In 1736, the French chemist, doctor and botanist Henri Louis Duhamel de Monceau was first able to obtain very pure soda from the water of soda lakes. He was able to establish that soda contains the chemical element “Natr”. In Russia, even during the time of Peter the Great, soda was called “zoda” or “itch” and until 1860 it was imported from abroad. In 1864, the first soda plant using the technology of the Frenchman Leblanc appeared in Russia. It was thanks to the emergence of its factories that soda became more accessible and began its victorious path as a chemical, culinary and even medicinal product.
Chemical properties
Sodium bicarbonate is an acidic sodium salt of carbonic acid. Molecular weight (according to international atomic masses 1971) - 84.00.
Reaction with acids
Sodium bicarbonate reacts with acids to form a salt and carbonic acid, which immediately breaks down into carbon dioxide and water:
NaHCO 3 + HCl → NaCl + H 2 CO 3
H 2 CO 3 → H 2 O + CO 2
in cooking, the following reaction with acetic acid is more common, with the formation of sodium acetate:
NaHCO 3 + CH 3 COOH → CH 3 COONa + H 2 O + CO 2
Soda dissolves well in water. An aqueous solution of baking soda has a slightly alkaline reaction. The hissing of soda is the result of the release of carbon dioxide CO 2 as a result of chemical reactions.
Thermal decomposition
At a temperature of 60° C, sodium bicarbonate decomposes into sodium carbonate, carbon dioxide and water (the decomposition process is most effective at 200° C):
2NaHCO 3 → Na 2 CO 3 + H 2 O + CO 2
With further heating to 1000° C (for example, when extinguishing a fire with powder systems), the resulting sodium carbonate decomposes into carbon dioxide and sodium oxide:
Na 2 CO 3 → Na 2 O + CO 2 .
physical and chemical indicators
Sodium bicarbonate is a white crystalline powder with an average crystal size of 0.05 - 0.20 mm. The molecular weight of the compound is 84.01, the density is 2200 kg/m³, and the bulk density is 0.9 g/cm³. The heat of dissolution of sodium bicarbonate is estimated at 205 kJ (48.8 kcal) per 1 kg of NaHCO 3, the heat capacity reaches 1.05 kJ/kg.K (0.249 kcal/kg.°C).
Sodium hydrogen carbonate is thermally unstable and, when heated, decomposes to form solid sodium carbonate and release carbon dioxide, as well as water into the gas phase:
2NaHCO 3 (tv.) ↔ Na 2 CO 3 (tv.) + CO 2 (g.) + H 2 O (steam) - 126 kJ (- 30 kcal) Aqueous solutions of sodium bicarbonate decompose similarly:
2NaHCO 3 (r.) ↔ Na 2 CO 3 (r.) + CO 2 (g.) + H 2 O (steam) - 20.6 kJ (- 4.9 kcal) An aqueous solution of sodium bicarbonate has a slightly alkaline character , and therefore has no effect on animal and plant tissues. The solubility of sodium bicarbonate in water is low and with increasing temperature it increases slightly: from 6.87 g per 100 g of water at 0 ° C to 19.17 g per 100 g of water at 80 ° C.
Due to low solubility, the density of saturated aqueous solutions of sodium bicarbonate differs relatively little from the density of pure water.
Boiling point (decomposes): 851°C;
Melting point: 270° C;
Density: 2.159 g/cm³;
Solubility in water, g/100 ml at 20° C: 9.
Application
Sodium bicarbonate (bicarbonate) is used in the chemical, food, light, medical, pharmaceutical industries, non-ferrous metallurgy, and is supplied to retail.
Registered as a food additive E500.
Widely used in:
- chemical industry - for the production of dyes, foam plastics and other organic products, fluoride reagents, household chemicals, fillers in fire extinguishers, for separating carbon dioxide, hydrogen sulfide from gas mixtures (gas is absorbed in a bicarbonate solution at elevated pressure and low temperature, the solution is restored when heated and low blood pressure).
- light industry - in the production of sole rubber and artificial leather, tanning (tanning and neutralizing leather).
- textile industry (finishing of silk and cotton fabrics). The use of sodium bicarbonate in the production of rubber products is also due to the release of CO 2 when heated, which helps give the rubber the necessary porous structure.
- food industry - bakery, confectionery production, beverage preparation.
- medical industry - for the preparation of injection solutions, anti-tuberculosis drugs and antibiotics.
- metallurgy - during the precipitation of rare earth metals and ore flotation.
Cooking
The main use of baking soda is cooking, where it is used mainly as a main or additional leavening agent in baking (as it releases carbon dioxide when heated), in the manufacture of confectionery products, in the production of carbonated drinks and artificial mineral waters, alone or as part of complex leavening agents ( for example, baking powder, mixed with ammonium carbonate), for example, in biscuit and shortbread dough. This is due to the ease of its decomposition at 50-100° C.
Baking soda, used primarily in the making of small cookies, pastry crumbs, cake sheets and puff pastries. In the last quarter of the 19th century. Its use in confectionery began, initially only in France and Germany, and only at the very end of the 19th century and at the beginning of the 20th century - also in Russia.
The use of soda opened the way to the factory production of modern cookies - stamped cookies. At the same time, many old types of cookies - sponge, puff, crushed, gingerbread, puffed, meringue - have become a thing of the past, disappearing not only from public use, but also from home use.
Soda is a necessary everyday assistant in the kitchen for washing dishes, canning containers, and some fruits and berries before drying. It has the property of neutralizing and killing odors.
It is a mistake to think that soda is a spice only for confectionery. In addition to confectionery production, soda is also used for the preparation of English marmalades, in minced meat for dishes of Moldavian, Romanian and Uzbek cuisine (potassium soda) and in the preparation of drinks. The amounts of soda added to all of the listed products are extremely small - from “at the tip of a knife” to a pinch and a quarter of a teaspoon. In drinks with soda, its share is much higher - half and a full teaspoon per liter of liquid. For confectionery and other purposes, soda is added as prescribed in recipes, usually in very small doses. Store it in an airtight container and take it with a dry object.
The production of soda industrially has provided ample opportunities for the preparation of many types of modern confectionery products in European countries. For a long time, Russia followed the traditional path, preferring yeast and other types of dough.
In Russia, until the second half of the 19th century, soda was not used at all in baking and confectionery. And at the very end of the 19th century, products of this kind were produced most of all in Ukraine and Poland, as well as in the Baltic states. The Russian population, accustomed from time immemorial to natural types of dough - either yeast, sourdough, or honey-egg, where artificial chemicals were not used as a lifting agent, but gases naturally occurring during baking were used as a result of the interaction of products such as honey ( sugar), eggs, sour cream, alcohol (vodka) or wine vinegar - soda cookies had extremely low popularity and low demand.
Confectionery products made with soda were considered “German” and were ignored both for purely culinary and taste reasons and for “patriotic” reasons.
In addition, Russian national confectionery products - honey gingerbreads and gingerbreads, glazed pearls and nuts boiled in honey - had such a uniquely excellent taste that they successfully competed with Western European ones, more refined in form, but “flimsy” in terms of satiety and good quality. and the taste of French biscuits, where the attractiveness was achieved not by the special nature of the dough, but by the use of exotic spices, mainly vanilla.
Apart from confectionery, soda has never been used in Russian cuisine and is actually not used to this day. Meanwhile, in the Baltics, Moldova, Romania, and the Balkans, soda is used as a leavening agent in a number of dishes prepared by frying. So, soda is added to a variety of semi-dough fried dishes: potato pancakes, which also includes wheat flour; a variety of pancakes, sour cream flatbreads and donuts, cheesecakes made from a combination of cottage cheese and flour, as well as minced meat, if they consist only of meat and onions, without adding flour components (flour, white bread, breadcrumbs). Such raw minced meat (beef, pork) is left with a soda additive to stand in the refrigerator for several hours, and then “sausages” are easily formed from this minced meat, which are quickly (in 10-15 minutes) grilled in the oven of any home stove (gas , wood or electric).
A similar use of soda in minced meat is also known in Armenian cuisine, with the only difference that in such cases the minced meat is not left to stand, but is immediately subjected to intensive beating with the addition of a few drops (5-8) of cognac, and actually turns into a meat soufflé used for preparing various national dishes (mainly kalolaks).
In English-speaking countries of Europe and America (England, Scotland, the East Coast of the USA and Canada), soda is used as an indispensable additive in citrus fruit jam (oranges, pampelmoses, lemons, grapefruits), as well as for the preparation of candied fruits. As a result, a special boilability of citrus fruits and their hard peels is achieved, turning such jam into a kind of thick marmalade, and at the same time the degree of unpleasant bitterness, always present in the peel of citrus fruits, is reduced (but does not disappear completely!). Orange peels, which form a kind of ballast for us, waste from eating these fruits, with the help of soda become valuable raw materials for producing aromatic, highly nutritious marmalade.
In Central Asian cuisines, soda is used in the preparation of non-confectionery types of simple dough in order to give it special elasticity and turn it into stretchable dough without the use of vegetable oil, as is customary in Southern European, Mediterranean and Balkan cuisines. In Central Asia, pieces of simple unleavened dough, after the usual half-hour resting, are moistened with a small amount of water in which 0.5 teaspoon of salt and 0.5 teaspoon of soda are dissolved, and then they are stretched by hand into the thinnest noodles (the so-called Dungan noodles), which has a delicate, pleasant taste and is used to prepare national dishes (lagman, monpara, shima, etc.).
Soda, as a tiny additive to any food during the cooking process, and specifically during heat treatment, is added in many national cuisines, given that in some cases this gives not only an unexpected taste effect, but also usually cleanses food raw materials and the entire dish from various random off-odors and tastes.
In general, the role of soda in the kitchen, even beyond the culinary process, is very significant. After all, without soda, it is practically impossible to perfectly clean dining and kitchen enamel, porcelain, glass and earthenware dishes, as well as kitchen tools and equipment from foreign odors and various deposits and patina. Soda is especially indispensable and necessary when cleaning tea utensils - teapots and cups from the tea deposits and films that form on their walls.
It is equally necessary to use soda when washing dishes in which fish was cooked in order to fight off the fishy smell. Usually they do the following: a persistent fishy smell is fought off by wiping the dishes with onions, and then the onion smell is destroyed (washed off) by cleaning the dishes with soda.
In a word, soda is an indispensable component of kitchen production, and a good kitchen cannot do without it. Moreover, its absence in the arsenal of a cook or housewife immediately becomes noticeable, for it binds the one who works at the stove or at the cutting table in many of his actions.
Modern environmental circumstances have given rise to another new use of soda in the kitchen as a means of improving the quality of vegetable raw materials. You can, for example, recommend washing all processed but not yet chopped vegetables - before placing them in a cauldron or frying pan - in a solution of soda in water. Or add one or two teaspoons of soda to already peeled potatoes, filled with cold water and intended for boiling or mashing. This will not only cleanse the potatoes of the chemicals that were used during their cultivation, but will also make the product itself lighter, cleaner, more beautiful, and will remove all odors acquired during transportation or improper storage, as well as spoilage. Once cooked, the potatoes themselves will become crumbly and tasty. Thus, the use of soda before cooking, during cold processing (then the product is thoroughly washed with cold water), can improve the quality of vegetable food raw materials, in particular starchy vegetables, root vegetables and leafy crops (cabbage, lettuce, spinach, parsley, etc. .).
Soda has taken the place of the alkaline agent so firmly that nothing has yet been able to move it from this position. Baking soda can act as a leavening agent in two ways. Firstly, it decomposes when heated according to the reaction:
2NaHCO 3 (soda) → Na 2 CO 3 (salt) + H 2 O (water) + CO 2 (carbon dioxide).
And in this case, if you add an excessive amount of soda to the shortbread dough, in a short baking time it may not have time to thermally decompose without leaving a residue and the cookies or cake will get an unpleasant “soda” taste.
Just like potash, soda reacts with acids contained in the dough or added there artificially:
NaHCO 3 (soda) + R-COOH (acid) → R-COONa (salt) + H 2 O (water) + CO 2 (carbon dioxide)
Many different branded bags and their availability do not cancel out the fun for young chemists - making their own baking powder.
proportional composition of such a traditional powder:
2 parts sour tartar salt,
1 part baking soda,
1 part starch or flour.
Medicine
Everyone knows what soda looks like - it is a white powder that absorbs water and dissolves well in it. But few people know about the amazing healing properties of this “simple” substance. Meanwhile, soda - sodium bicarbonate - is one of the main ingredients of our blood. The results of a study of the effect of soda on the human body exceeded all expectations. It turned out that soda is able to equalize the acid-base balance in the body, restore metabolism in cells, improve the absorption of oxygen by tissues, and also prevent the loss of vital potassium. Baking soda helps with heartburn, seasickness, colds, heart disease and headaches, and skin diseases. As you can see, soda is a first aid medicine.
A solution of baking soda is used as a weak antiseptic for rinsing, as well as a traditional acid-neutralizing remedy for heartburn and stomach pain (modern medicine does not recommend its use due to side effects, including “acid rebound”) or to eliminate acidosis, etc.
Baking soda is used to treat diseases associated with high acidity; a solution of baking soda is used to gargle and to wash the skin in case of acid contact.
Sodium bicarbonate (baking soda) may slow the progression of chronic kidney disease. This conclusion was reached by scientists from the Royal London Hospital, UK. They studied 134 people with advanced chronic kidney disease and metabolic acidosis.
One group of subjects received the usual treatment, and the second, in addition to traditional treatment, received a small amount of baking soda daily in the form of tablets. In those patients who drank sodium bicarbonate, kidney function deteriorated 2/3 slower than in others.
Rapid progression of kidney disease was observed in only 9% of experimental subjects from the “soda group” versus 45% of subjects treated traditionally. In addition, those who took soda were less likely to develop end-stage renal disease, which requires dialysis. It is noteworthy that the increase in sodium bicarbonate in the body did not cause an increase in blood pressure in patients.
Baking soda is an inexpensive and effective treatment for chronic kidney disease. However, the researchers caution: taking soda should be under the supervision of a doctor, who must correctly calculate the dosage for the patient.
The healing properties of baking soda
Previously, sodium bicarbonate was used very widely (like other alkalis) as an antacid for high acidity of gastric juice, gastric and duodenal ulcers. When taken orally, baking soda quickly neutralizes the hydrochloric acid of gastric juice and has a pronounced antacid effect. However, the use of soda is not only about brilliantly washed dishes and getting rid of heartburn. Baking soda takes its rightful place in the home medicine cabinet.
Like the ancient Egyptians, who obtained natural soda from lake waters by evaporation, people also used other properties of soda. It has neutralizing qualities and is used in medical practice to treat gastritis with high acidity. Capable of killing germs, used as a disinfectant: soda is used for inhalation, rinsing, and skin cleansing.
Soda is also widely used in healthcare.
Prevention of caries.
Acids formed in the mouth as a result of bacterial activity destroy tooth enamel. These acids can be neutralized by rinsing your mouth with a baking soda solution several times a day. You can do it differently: wet your toothbrush with water, dip it in baking soda and brush your teeth. Baking soda, in addition, has a slight abrasive effect: it will polish your teeth without damaging the enamel.
From unpleasant foot odor.
Added soda to foot bath water neutralizes the acids produced by bacteria, which give the feet an unpleasant odor. Baking soda will also help eliminate the pungent odor of armpit sweat.
For insect bites.
Do not scratch the bites of mosquitoes and other bloodsuckers until they bleed. It is better to prepare a porridge mixture of water and soda and apply it to the bite site. Soda gruel will also relieve itching caused by chickenpox or skin contact with hogweed and nettle.
For diaper rash.
Soda lotions significantly improve the condition of babies with diaper rash. They reduce itching and speed up skin healing.
For cystitis.
Pathogenic bacteria live in the bladder in a slightly acidic environment. If your bladder has fallen victim to an infection, the ideal after-dinner drink for you is a fizzy cocktail of baking soda and water.
For sunburn.
Add some baking soda to a warm bath; it will soften the water, turning it into a soothing lotion for irritated skin.
For sore throat.
Stir 0.5 tsp. spoons of soda in a glass of water and gargle with the prepared solution every 4 hours: it neutralizes acids that cause pain. Rinsing your mouth with this solution will help relieve inflammation of the oral mucosa.
For bad breath.
When combined with hydrogen peroxide, baking soda has a powerful oxidizing effect and destroys bacteria that cause bad breath. Add 1 table. spoon of baking soda into a glass of hydrogen peroxide solution (2-3%) and rinse your mouth.
For a cold.
It is useful to do inhalation. To do this, you can take a small kettle and boil 1 glass of water in it with 1 teaspoon. spoon of soda. Make a tube out of hard paper, put it on the spout of the kettle and inhale the steam for 10-15 minutes. This inhalation is very helpful in removing mucus.
To expectorate viscous sputum, drink 1/2 cup of warm water on an empty stomach, in which 0.5 teaspoon is dissolved, 2 times a day. spoons of soda and a pinch of salt.
For frequent migraines.
Every day, take a solution of boiled water and baking soda. On the 1st day, 30 minutes before lunch, drink 1 glass of solution (0.5 teaspoon of soda + water), 2nd day - 2 glasses, etc., bringing up to 7 glasses. Then reduce the dose in the reverse order.
Other.
For rhinitis, stomatitis, laryngitis, conjunctivitis, use a 0.5-2% soda solution.
To disinfect the oral mucosa, it is useful to rinse your mouth with a weak solution (soda - 85 g, salt - 85 g, urea - 2.5 g) after eating.
Smoking remedy: rinse your mouth with a solution of baking soda (1 tablespoon per 200 ml of water).
For dry skin, dry dermatitis, ichthyosis and psoriasis, medicinal baths are useful (soda - 35 g, magnesium carbonate - 20 g, magnesium perborate - 15 g). The water temperature should not be higher than 38-39° C, first you just need to sit in a warm bath, then gradually increase the temperature. The duration of the bath is 15 minutes.
Firefighting
Sodium bicarbonate is part of the powder used in powder fire extinguishing systems, utilizing heat and displacing oxygen from the combustion source with the released carbon dioxide.
Equipment cleaning. Abrasive blast cleaning technology (ABL)
Equipment and surfaces are cleaned of various coatings and contaminants using abrasive blast cleaning (ABL) technology. Sodium bicarbonate (baking soda, sodium bicarbonate, sodium bicarbonate, NaHCO 3, sodium hydrogen carbonate) is used as an abrasive.
ASO technology using sodium bicarbonate is a new effective way to clean equipment using a “soft” abrasive. The abrasive is driven by compressed air produced by a compressor. This method has gained commercial acceptance and has been widely used in Europe and the USA for 25 years due to its versatility and economic feasibility.
Equipment surface treatment is similar to conventional sandblasting. The difference is that soda particles are a “soft” abrasive material, that is, they do not damage the surface itself.
Principle:
A fragile particle of acidic sodium carbonate explodes upon contact with the surface being cleaned.
The energy released by this flash removes contaminants from the surface being cleaned. Abrasive soda particles are completely broken into fine dust, which easily scatters in different directions perpendicular to the fall, increasing the cleaning effect. For dust suppression purposes, soda blast cleaning of equipment is usually performed using humidification, that is, hydro-abrasive blast cleaning (HABL) of the equipment. Sodium carbonate dissolves in water. Therefore, the used abrasive will be dissolved or can be washed off after cleaning.
This is different from quartz sand, which cuts off the coating. Quartz sand also erases part of the surface being cleaned, which soda leaves virtually unharmed. There are still many differences between these types of equipment cleaning, but they are already a consequence of the properties of abrasives.
Soluble sodium bicarbonate abrasives are specially formulated for abrasive blast cleaning of equipment. The free-flowing properties of abrasives reduce the flow density associated with the poor fluidity of conventional sodium carbonate.
- Isolating the contour of a liquid drop in the problem of determining surface tension Isolating the contour of a drop
- Method for producing calcium peroxide
- Feo color. Chemical properties. Refractory black pyrophoric powder, insoluble in water. Methods for obtaining iron
- Sodium thiosulfate Natrii thiosulfas (ln) Thiosulfate chemical formula
- Rhodanide method for determining iron
- Baking soda: formula, application
- Carbonates. Baking soda formula. Baking soda: formula, application Safety of baking soda for humans
- Description of the disease, signs, treatment
- Liver diseases: causes, types, symptoms and prevention What is a virus in the liver
- Helminthic infestation: causes, symptoms, treatment, prevention
- Folk remedies
- Food fermentation and its importance
- Study of pharmacokinetics and bioavailability - journal of pharmacokinetics and pharmacodynamics High performance liquid chromatography
- Test work chloroform Chloroform at home
- Noble gases and their properties Noble gases what causes the inertness of these gases
- Raman and NIR spectroscopy Problems of NIR spectrometry and how to solve them
- Side specialties of tantalum
- What is tantalum and where is it used?
- Molecular effects of enzyme action
- Cervical dysplasia: first signs, symptoms and treatment