Feo color. Chemical properties. Refractory black pyrophoric powder, insoluble in water. Methods for obtaining iron
68. Iron compounds
Iron (II) oxide FeO– a black crystalline substance, insoluble in water and alkalis. FeO matches the base Fe(OH)2.
Receipt. Iron (II) oxide can be obtained by incomplete reduction of magnetic iron ore with carbon (II) oxide:
Chemical properties. It is the main oxide. Reacting with acids, it forms salts:
Iron (II) hydroxide Fe(OH)2- white crystalline substance.
Receipt. Iron (II) hydroxide is obtained from divalent iron salts under the action of alkali solutions:
Chemical properties. Basic hydroxide. Reacts with acids:
In air, Fe(OH)2 is oxidized to Fe(OH)3:
Iron(III) oxide Fe2O3– a brown substance, found in nature in the form of red iron ore, insoluble in water.
Receipt. When firing pyrite:
Chemical properties. Exhibits weak amphoteric properties. When interacting with alkalis, it forms salts:
Iron (III) hydroxide Fe(OH)3– a red-brown substance, insoluble in water and excess alkali.
Receipt. Obtained by the oxidation of iron (III) oxide and iron (II) hydroxide.
Chemical properties. It is an amphoteric compound (with a predominance of basic properties). Precipitates under the action of alkalis on ferric iron salts:
Ferrous salts obtained by reacting metallic iron with appropriate acids. They are highly hydrolyzed, which is why their aqueous solutions are energetic reducing agents:
When heated above 480 °C, it decomposes, forming oxides:
When alkalis act on iron (II) sulfate, iron (II) hydroxide is formed:
Forms crystalline hydrate - FeSO4?7Н2О (iron sulfate). Iron (III) chloride FeCl3 – dark brown crystalline substance.
Chemical properties. Let's dissolve in water. FeCl3 exhibits oxidizing properties.
Reducing agents - magnesium, zinc, hydrogen sulfide, oxidize without heating.
The human body contains about 5 g of iron, most of it (70%) is part of blood hemoglobin.
Physical properties
In its free state, iron is a silvery-white metal with a grayish tint. Pure iron is ductile and has ferromagnetic properties. In practice, iron alloys - cast iron and steel - are usually used.
Fe is the most important and most abundant element of the nine d-metals of the Group VIII subgroup. Together with cobalt and nickel it forms the “iron family”.
When forming compounds with other elements, it often uses 2 or 3 electrons (B = II, III).
Iron, like almost all d-elements of group VIII, does not exhibit a higher valency equal to the group number. Its maximum valency reaches VI and appears extremely rarely.
The most typical compounds are those in which the Fe atoms are in oxidation states +2 and +3.
Methods for obtaining iron
1. Technical iron (alloyed with carbon and other impurities) is obtained by carbothermic reduction of its natural compounds according to the following scheme:

Recovery occurs gradually, in 3 stages:
1) 3Fe 2 O 3 + CO = 2Fe 3 O 4 + CO 2
2) Fe 3 O 4 + CO = 3FeO + CO 2
3) FeO + CO = Fe + CO 2
The cast iron resulting from this process contains more than 2% carbon. Subsequently, cast iron is used to produce steel - iron alloys containing less than 1.5% carbon.
2. Very pure iron is obtained in one of the following ways:
a) decomposition of Fe pentacarbonyl
Fe(CO) 5 = Fe + 5СО
b) reduction of pure FeO with hydrogen
FeO + H 2 = Fe + H 2 O
c) electrolysis of aqueous solutions of Fe +2 salts
FeC 2 O 4 = Fe + 2CO 2
iron(II) oxalate
Chemical properties
Fe is a metal of medium activity and exhibits general properties characteristic of metals.
A unique feature is the ability to “rust” in humid air:
In the absence of moisture with dry air, iron begins to react noticeably only at T > 150°C; upon calcination, “iron scale” Fe 3 O 4 is formed:
3Fe + 2O 2 = Fe 3 O 4
Iron does not dissolve in water in the absence of oxygen. At very high temperatures, Fe reacts with water vapor, displacing hydrogen from water molecules:
3 Fe + 4H 2 O(g) = 4H 2
The mechanism of rusting is electrochemical corrosion. The rust product is presented in a simplified form. In fact, a loose layer of a mixture of oxides and hydroxides of variable composition is formed. Unlike the Al 2 O 3 film, this layer does not protect iron from further destruction.
Types of corrosion

Protecting iron from corrosion

1. Interaction with halogens and sulfur at high temperatures.
2Fe + 3Cl 2 = 2FeCl 3
2Fe + 3F 2 = 2FeF 3
Fe + I 2 = FeI 2
Compounds are formed in which the ionic type of bond predominates.
2. Interaction with phosphorus, carbon, silicon (iron does not directly combine with N2 and H2, but dissolves them).
Fe + P = Fe x P y
Fe + C = Fe x C y
Fe + Si = Fe x Si y
Substances of variable composition are formed, such as berthollides (the covalent nature of the bond predominates in the compounds)
3. Interaction with “non-oxidizing” acids (HCl, H 2 SO 4 dil.)
Fe 0 + 2H + → Fe 2+ + H 2
Since Fe is located in the activity series to the left of hydrogen (E° Fe/Fe 2+ = -0.44 V), it is capable of displacing H 2 from ordinary acids.
Fe + 2HCl = FeCl 2 + H 2
Fe + H 2 SO 4 = FeSO 4 + H 2
4. Interaction with “oxidizing” acids (HNO 3, H 2 SO 4 conc.)
Fe 0 - 3e - → Fe 3+
Concentrated HNO 3 and H 2 SO 4 “passivate” iron, so at ordinary temperatures the metal does not dissolve in them. With strong heating, slow dissolution occurs (without releasing H 2).
In the section HNO 3 iron dissolves, goes into solution in the form of Fe 3+ cations and the acid anion is reduced to NO*:
Fe + 4HNO 3 = Fe(NO 3) 3 + NO + 2H 2 O
Very soluble in a mixture of HCl and HNO 3
5. Relation to alkalis
Fe does not dissolve in aqueous solutions of alkalis. It reacts with molten alkalis only at very high temperatures.
6. Interaction with salts of less active metals
Fe + CuSO 4 = FeSO 4 + Cu
Fe 0 + Cu 2+ = Fe 2+ + Cu 0
7. Reaction with gaseous carbon monoxide (t = 200°C, P)
Fe (powder) + 5CO (g) = Fe 0 (CO) 5 iron pentacarbonyl
Fe(III) compounds
Fe 2 O 3 - iron (III) oxide.
Red-brown powder, n. R. in H 2 O. In nature - “red iron ore”.
Methods of obtaining:
1) decomposition of iron (III) hydroxide
2Fe(OH) 3 = Fe 2 O 3 + 3H 2 O
2) pyrite firing
4FeS 2 + 11O 2 = 8SO 2 + 2Fe 2 O 3
3) nitrate decomposition
Chemical properties
Fe 2 O 3 is a basic oxide with signs of amphotericity.
I. The main properties are manifested in the ability to react with acids:
Fe 2 O 3 + 6H + = 2Fe 3+ + ZN 2 O
Fe 2 O 3 + 6HCI = 2FeCI 3 + 3H 2 O
Fe 2 O 3 + 6HNO 3 = 2Fe(NO 3) 3 + 3H 2 O
II. Weak acid properties. Fe 2 O 3 does not dissolve in aqueous solutions of alkalis, but when fused with solid oxides, alkalis and carbonates, ferrites form:
Fe 2 O 3 + CaO = Ca(FeO 2) 2
Fe 2 O 3 + 2NaOH = 2NaFeO 2 + H 2 O
Fe 2 O 3 + MgCO 3 = Mg(FeO 2) 2 + CO 2
III. Fe 2 O 3 - feedstock for the production of iron in metallurgy:
Fe 2 O 3 + ZS = 2Fe + ZSO or Fe 2 O 3 + ZSO = 2Fe + ZSO 2
Fe(OH) 3 - iron (III) hydroxide
Methods of obtaining:
Obtained by the action of alkalis on soluble Fe 3+ salts:
FeCl 3 + 3NaOH = Fe(OH) 3 + 3NaCl
At the time of preparation, Fe(OH) 3 is a red-brown mucous-amorphous sediment.
Fe(III) hydroxide is also formed during the oxidation of Fe and Fe(OH) 2 in moist air:
4Fe + 6H 2 O + 3O 2 = 4Fe(OH) 3
4Fe(OH) 2 + 2H 2 O + O 2 = 4Fe(OH) 3
Fe(III) hydroxide is the end product of the hydrolysis of Fe 3+ salts.
Chemical properties
Fe(OH) 3 is a very weak base (much weaker than Fe(OH) 2). Shows noticeable acidic properties. Thus, Fe(OH) 3 has an amphoteric character:
1) reactions with acids occur easily:
2) fresh precipitate of Fe(OH) 3 dissolves in hot conc. solutions of KOH or NaOH with the formation of hydroxo complexes:
Fe(OH) 3 + 3KOH = K 3
In an alkaline solution, Fe(OH) 3 can be oxidized to ferrates (salts of iron acid H 2 FeO 4 not released in the free state):
2Fe(OH) 3 + 10KOH + 3Br 2 = 2K 2 FeO 4 + 6KBr + 8H 2 O
Fe 3+ salts
The most practically important are: Fe 2 (SO 4) 3, FeCl 3, Fe(NO 3) 3, Fe(SCN) 3, K 3 4 - yellow blood salt = Fe 4 3 Prussian blue (dark blue precipitate)
b) Fe 3+ + 3SCN - = Fe(SCN) 3 thiocyanate Fe(III) (blood red solution)
Iron forms two oxides, in which it exhibits valencies II and III and oxidation states (+2) and (+3), respectively.
DEFINITION
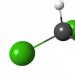
Iron(II) oxide under normal conditions it is a black powder (Fig. 1), decomposing upon moderate heating and forming again from decomposition products upon further heating.
After calcination it is chemically inactive. Pyrophoric in powder form. Does not react with cold water. Exhibits amphoteric properties (with a predominance of basic ones). Easily oxidized by oxygen. Reduced by hydrogen and carbon.
Rice. 1. Iron (II) oxide. Appearance.
DEFINITION
It is a red-brown solid in the case of the trigonal modification or dark brown in the case of the cubic modification, which is the most reactive (Fig. 1).
Thermally stable. Melting point 1562 o C.

Rice. 1. Iron (III) oxide.
Does not react with water, ammonia hydrate. Shows amphoteric properties, reacts with acids and alkalis. Reduced by hydrogen, carbon monoxide, iron.
Chemical formula of iron oxide
The chemical formula of iron (II) oxide is FeO, and the chemical formula of iron (III) oxide is Fe 2 O 3. The chemical formula shows the qualitative and quantitative composition of the molecule (how many and what atoms are present in it). Using the chemical formula, you can calculate the molecular mass of a substance (Ar(Fe) = 56 amu, Ar(O) = 16 amu):
Mr(FeO) = Ar(Fe) + Ar(O);
Mr(FeO) = 56 + 16 = 72.
Mr(Fe 2 O 3) = 2×Ar(Fe) + 3×Ar(O);
Mr(Fe 2 O 3) = 2×56 + 3×16 = 58 + 48 = 160.
Structural (graphic) formula of iron oxide
The structural (graphic) formula of a substance is more visual. It shows how atoms are connected to each other within a molecule. Below are the graphic formulas of iron oxides (a - FeO, b - Fe 2 O 3):

Examples of problem solving
EXAMPLE 1
| Exercise | Having analyzed the substance, it was found that its composition includes: sodium with a mass fraction of 0.4207 (or 42.07%), phosphorus with a mass fraction of 0.189 (or 18.91%), oxygen with a mass fraction of 0.3902 (or 39 .02%). Find the formula of the compound. |
| Solution | Let us denote the number of sodium atoms in the molecule by “x”, the number of phosphorus atoms by “y” and the number of oxygen atoms by “z”. Let's find the corresponding relative atomic masses of the elements sodium, phosphorus and oxygen (the values of the relative atomic masses taken from D.I. Mendeleev's Periodic Table are rounded to whole numbers). Ar(Na) = 23; Ar(P) = 31; Ar(O) = 16. We divide the percentage content of elements into the corresponding relative atomic masses. Thus we will find the relationship between the number of atoms in the molecule of the compound: Na:P:O = 42.07/39: 18.91/31: 39.02/16; Na:P:O = 1.829: 0.61: 2.43. Let’s take the smallest number as one (i.e., divide all numbers by the smallest number 0.61): 1,829/0,61: 0,61/0,61: 2,43/0,61; Consequently, the simplest formula for the compound of sodium, phosphorus and oxygen is Na 3 PO 4. This is sodium phosphate. |
| Answer | Na3PO4 |
EXAMPLE 2
| Exercise | The molar mass of the nitrogen-hydrogen compound is 32 g/mol. Determine the molecular formula of a substance whose mass fraction of nitrogen is 85.7%. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: ω (X) = n × Ar (X) / M (HX) × 100%. Let's calculate the mass fraction of hydrogen in the compound: ω(H) = 100% - ω(N) = 100% - 85.7% = 14.3%. Let us denote the number of moles of elements included in the compound as “x” (nitrogen), “y” (hydrogen). Then, the molar ratio will look like this (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers): x:y = ω(N)/Ar(N) : ω(H)/Ar(H); x:y= 85.7/14: 14.3/1; x:y= 6.12: 14.3= 1: 2. This means that the simplest formula for combining nitrogen with hydrogen will be NH 2 and a molar mass of 16 g/mol. To find the true formula of an organic compound, we find the ratio of the resulting molar masses: M substance / M(NH 2) = 32 / 16 = 2. This means that the indices of nitrogen and hydrogen atoms should be 2 times higher, i.e. the formula of the substance will be N 2 H 4. This is hydrazine. |
| Answer | N2H4 |
Iron oxides are compounds of iron and oxygen.
The most famous are three iron oxides: iron oxide (II) - FeO, iron (III) oxide – Fe 2 O 3 and iron (II, III) oxide – Fe 3 O 4.
Iron(II) oxide

The chemical formula of ferrous oxide is FeO . This connection is black in color.
FeO Reacts easily with dilute hydrochloric acid and concentrated nitric acid.
FeO + 2HCl → FeCl 2 + H 2 O
FeO + 4HNO 3 → Fe(NO 3) 3 + NO 2 + 2H 2 O
It does not react with water or salts.
When interacting with hydrogen at a temperature of 350 o C and coke at a temperature above 1000 o C, it is reduced to pure iron.
FeO +H 2 → Fe + H 2 O
FeO +C → Fe + CO
Iron (II) oxide is obtained in different ways:
1. As a result of the reduction reaction of ferric oxide with carbon monoxide.
Fe 2 O 3 + CO → 2 FeO + CO 2
2. Heating iron with low oxygen pressure
2Fe + O 2 → 2 FeO
3. Decomposing ferrous oxalate in vacuum
FeC 2 O 4 → FeO +CO + CO 2
4. Interaction of iron with iron oxides at a temperature of 900-1000 o
Fe + Fe 2 O 3 → 3 FeO
Fe + Fe 3 O 4 → 4 FeO
In nature, ferrous oxide exists as the mineral wustite.
In industry it is used in the smelting of cast iron in blast furnaces, in the process of blackening (bluing) of steel. It is found in dyes and ceramics.
Iron(III) oxide

Chemical formula Fe2O3 . This is a compound of ferric iron with oxygen. It is a red-brown powder. Hematite is found in nature as a mineral.
Fe2O3 has other names: iron oxide, red lead, crocus, pigment red 101, food coloringE172 .
Does not react with water. Can interact with both acids and alkalis.
Fe 2 O 3 + 6HCl → 2 FeCl 3 + 3H 2 O
Fe 2 O 3 + 2NaOH → 2NaFeO 2 + H 2 O
Iron (III) oxide is used for painting building materials: brick, cement, ceramics, concrete, paving slabs, linoleum. It is added as a dye to paints and enamels, and to printing inks. Iron oxide is used as a catalyst in the production of ammonia. In the food industry it is known as E172.
Iron (II, III) oxide

Chemical formula Fe3O4 . This formula can be written in another way: FeO Fe 2 O 3.
It is found in nature as the mineral magnetite, or magnetic iron ore. It is a good conductor of electric current and has magnetic properties. Formed when iron burns and when superheated steam acts on iron.
3Fe + 2 O 2 → Fe 3 O 4
3Fe + 4H 2 O → Fe 3 O 4 + 4H 2
Heating at a temperature of 1538 o C leads to its disintegration
2Fe 3 O 4 → 6FeO + O 2
Reacts with acids
Fe 3 O 4 + 8HCl → FeCl 2 + 2FeCl 3 + 4H 2 O
Fe 3 O 4 + 10HNO 3 → 3Fe(NO 3) 3 + NO 2 + 5H 2 O
Reacts with alkalis upon fusion
Fe 3 O 4 + 14NaOH → Na 3 FeO 3 + 2Na 5 FeO 4 + 7H 2 O
Reacts with oxygen in the air
4 Fe 3 O 4 + O 2 → 6Fe 2 O 3
Reduction occurs by reaction with hydrogen and carbon monoxide
Fe 3 O 4 + 4H 2 → 3Fe + 4H 2 O
Fe 3 O 4 + 4CO → 3Fe +4CO 2
Magnetic nanoparticles of Fe 3 O 4 oxide have found application in magnetic resonance imaging. They are also used in the production of magnetic media. Iron oxide Fe 3 O 4 is included in paints that are produced specifically for warships, submarines and other equipment. Electrodes are made from fused magnetite for some electrochemical processes.
- Isolating the contour of a liquid drop in the problem of determining surface tension Isolating the contour of a drop
- Method for producing calcium peroxide
- Feo color. Chemical properties. Refractory black pyrophoric powder, insoluble in water. Methods for obtaining iron
- Sodium thiosulfate Natrii thiosulfas (ln) Thiosulfate chemical formula
- Rhodanide method for determining iron
- Baking soda: formula, application
- Carbonates. Baking soda formula. Baking soda: formula, application Safety of baking soda for humans
- Description of the disease, signs, treatment
- Liver diseases: causes, types, symptoms and prevention What is a virus in the liver
- Helminthic infestation: causes, symptoms, treatment, prevention
- Folk remedies
- Food fermentation and its importance
- Study of pharmacokinetics and bioavailability - journal of pharmacokinetics and pharmacodynamics High performance liquid chromatography
- Test work chloroform Chloroform at home
- Noble gases and their properties Noble gases what causes the inertness of these gases
- Raman and NIR spectroscopy Problems of NIR spectrometry and how to solve them
- Side specialties of tantalum
- What is tantalum and where is it used?
- Molecular effects of enzyme action
- Cervical dysplasia: first signs, symptoms and treatment